1. An international research group consisting of NIMS and the Finnish University of Jyväskylä has discovered through its electrode-electrolyte system research that electron and proton (i.e., hydrogen ion) transfer mechanisms during oxygen reduction reactions (ORRs) on electrode surfaces vary depending on the types of cations dissolved in the electrolytic solution. These results suggest that the energy conversion efficiencies and selectivity of electrochemical systems (e.g., fuel cells and water electrolysis hydrogen production systems) can be improved by selecting optimal reaction pathways and that this could be achieved without using expensive electrode materials.
Credit: Ken Sakaushi, Tomoaki Kumeda
National Institute for Materials Science
1. An international research group consisting of NIMS and the Finnish University of Jyväskylä has discovered through its electrode-electrolyte system research that electron and proton (i.e., hydrogen ion) transfer mechanisms during oxygen reduction reactions (ORRs) on electrode surfaces vary depending on the types of cations dissolved in the electrolytic solution. These results suggest that the energy conversion efficiencies and selectivity of electrochemical systems (e.g., fuel cells and water electrolysis hydrogen production systems) can be improved by selecting optimal reaction pathways and that this could be achieved without using expensive electrode materials.
2. Most electrochemical reactions take place at the interface between an electrode and an electrolytic solution. It is therefore important to understand how the structures and characteristics of electrodes and electrolytes interact in controlling electrochemical reaction rates at their interfaces and in ascertaining reaction mechanisms. It had been known that ORR rates in fuel cells are influenced by the types and concentrations of ions dissolved in their electrolytes. However, detailed ORR pathways and how electrolytic ions influence these pathways had been unknown.
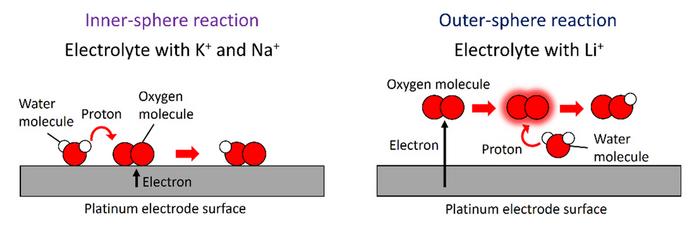
3. This research group recently investigated ORRs taking place at the interface between an alkaline electrolyte and a platinum electrode and discovered for the first time that electron and proton transfer mechanisms during ORRs vary depending on the types of cations dissolved in the electrolyte. Two types of ORR pathways take shape on the surface of a platinum electrode, each yielding different ORR products: inner-sphere (IS) and outer-sphere (OS) ORR pathways. In the IS ORR pathway, oxygen molecules in the electrolyte adsorb to the electrode surface, accepting an electron from the electrode. The molecular oxygen intermediates are then dissociated into atomic oxygen intermediates. By contrast, in the OS pathway, oxygen molecules accept electrons from the electrode at locations a slight distance from the electrode surface. In-depth analyses using high-precision electrochemical measurements and first-principles calculations revealed that the OS pathway tends to occur when the electrolyte contains lithium ions, while the IS pathway is more common with the electrolyte containing potassium and sodium ions—consistent with previously published findings. These differences in ORR pathways may be attributed to the ways in which these electrolytic cations interact with the electrode surface, water molecules and ORR intermediates.
4. This research found that ORR mechanisms can be controlled by changing electrolytic compositions in addition to the traditional approach of changing electrode material compositions. This discovery suggests that the energy conversion efficiency of electrocatalytic systems can be improved even without using expensive electrode materials. In addition, by improving the performance of both electrodes and electrolytes, it may be feasible to achieve even higher ORR efficiency and increase ORR pathway options.
***
5. This project was carried out by an international research group consisting of Tomoaki Kumeda (Research Fellow, International Center for Young Scientists (ICYS), NIMS), Ken Sakaushi (Principal Researcher, Research Center for Energy and Environmental Materials, NIMS) and the University of Jyväskylä.
6. This research was published in the online version of Angewandte Chemie International Edition, a journal of the German Chemical Society, on November 20, 2023.
Journal
Angewandte Chemie International Edition
DOI
10.1002/anie.202312841
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Cations Determine the Mechanism and Selectivity of Alkaline Oxygen Reduction Reaction on Pt(111)
Article Publication Date
20-Nov-2023




