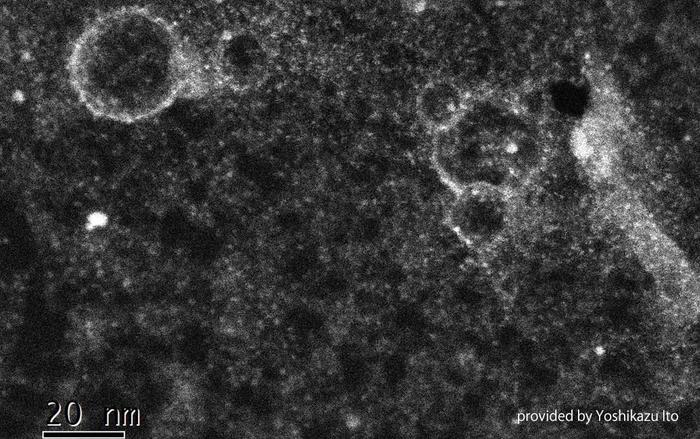
Tsukuba, Japan—For realizing carbon neutrality, the demand for the development of direct methanol/formic acid-fuel cell technology has been increasing. In this technology, methanol or formic acid is used as an e-fuel for generating electricity. The fuel cells generate electricity via proton transfer; however, conventional proton-exchange membranes suffer from the “crossover phenomenon,” where the fuel molecules are also transferred between anodes and cathodes. Thereafter, the fuel molecules are unnecessarily oxidized and the electrodes are deactivated. In this study, the researchers developed a new proton-exchange membrane comprising graphene sheets with 5-10 nm-diameter holes, which are chemically modified with sulfanilic functional groups affording sulfo groups around the holes. Owing to steric hindrance by the functional groups, the graphene membrane successfully suppresses the crossover phenomenon by blocking the penetration of the fuel molecules while maintaining high proton conductivity for the first time to the best of our knowledge.
Credit: University of Tsukuba
Tsukuba, Japan—For realizing carbon neutrality, the demand for the development of direct methanol/formic acid-fuel cell technology has been increasing. In this technology, methanol or formic acid is used as an e-fuel for generating electricity. The fuel cells generate electricity via proton transfer; however, conventional proton-exchange membranes suffer from the “crossover phenomenon,” where the fuel molecules are also transferred between anodes and cathodes. Thereafter, the fuel molecules are unnecessarily oxidized and the electrodes are deactivated. In this study, the researchers developed a new proton-exchange membrane comprising graphene sheets with 5-10 nm-diameter holes, which are chemically modified with sulfanilic functional groups affording sulfo groups around the holes. Owing to steric hindrance by the functional groups, the graphene membrane successfully suppresses the crossover phenomenon by blocking the penetration of the fuel molecules while maintaining high proton conductivity for the first time to the best of our knowledge.
To date, conventional approaches for inhibiting fuel-molecule migration involved an increase of the membrane thickness or sandwiching two-dimensional materials, which in turn reduced the proton conductivity. In this study, the researchers investigated structures that inhibit the migration of fuel molecules through electro-osmotic drag and steric hindrance. Consequently, they found that the sulfanilic-functionalized graphene membrane can remarkably suppress electrode degradation compared with the commercially-available Nafion membranes while maintaining the proton conductivity required for fuel cells.
Furthermore, simply pasting the graphene membrane onto a conventional proton-exchange membrane can suppress the crossover phenomenon. Thus, this study contributes to the development of advanced fuel cells as a new alternative for hydrogen-type fuel cells.
###
JST-PRESTO (JPMJPR2115), the Mukai science and technology foundation, the JSPS Grant-in-Aid for Scientific Research on Innovative Areas “Discrete Geometric Analysis for Materials Design” (Grant Numbers JP20H04628, JP20H04639), JSPS KAKENHI (Grant Numbers JP21H02037, JP21KK0091, JP23K17661), and a cooperative program (Proposal No. 202111-CRKEQ-0001) of CRDAM-IMR, Tohoku University.
Original Paper
Title of original paper:
Suppression of Methanol and Formate Crossover through Sulfanilic-functionalized Holey Graphene as Proton Exchange Membranes
Journal:
Advanced Science
DOI:
10.1002/advs.202304082
Correspondence
Assistant Professor Jeong Samuel
Associate Professor ITO, Yoshikazu
Institute of Pure and Applied Science, University of Tsukuba
Associate professor OHTO, Tatsuhiko
Graduate School of Engineering, Nagoya University
Assistant Professor NISHIUCHI, Tomohiko
Graduate School of Science, Osaka University
Related Link
Institute of Pure and Applied Sciences
Journal
Advanced Science
DOI
10.1002/advs.202304082
Article Title
Suppression of Methanol and Formate Crossover through Sulfanilic-Functionalized Holey Graphene as Proton Exchange Membranes
Article Publication Date
8-Sep-2023




