A groundbreaking study from the University of São Paulo (USP) unveils early neuroinflammation as a critical factor in the accelerated development of Alzheimer’s disease among individuals with Down syndrome. This discovery provides a new avenue for therapeutic intervention, significantly advancing our understanding of the pathological mechanisms underlying this debilitating neurodegenerative condition. With an estimated 90% of people with Down syndrome developing Alzheimer’s disease by age 70, the early detection and characterization of neuroinflammation mark a pivotal step in disease prevention and management.
Down syndrome, caused by the triplication of chromosome 21, leads to the overexpression of several genes, including the amyloid precursor protein (APP) gene. This genetic anomaly results in elevated production of beta-amyloid peptides, which aggregate into plaques—a hallmark of Alzheimer’s pathology. Until now, it was largely understood that beta-amyloid deposition initiates much of the neurodegenerative cascade. However, the recent nuclear medicine PET imaging study illuminates that neuroinflammation not only coexists but actually precedes and may drive the amyloid pathology in young adults with Down syndrome, beginning as early as their twenties.
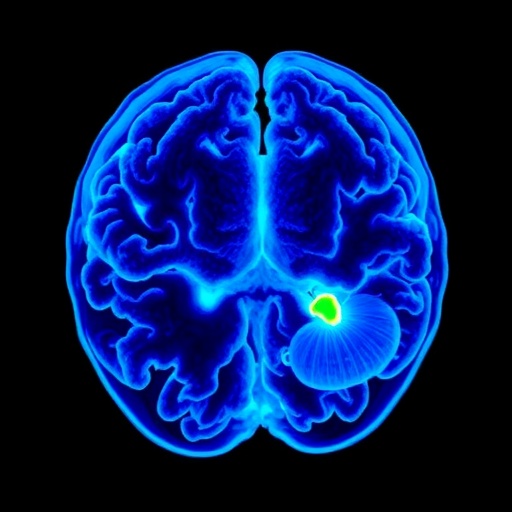
Utilizing advanced positron emission tomography (PET) techniques with novel radiopharmaceuticals, researchers mapped neuroinflammatory patterns across various brain regions in both Down syndrome and neurotypical individuals aged 20 to 50. Their technique uniquely allows real-time visualization of beta-amyloid plaque accumulation and inflammatory cell activity, chiefly involving microglia, the brain’s resident immune cells. The study revealed heightened neuroinflammation in frontal, temporal, occipital, and limbic regions among Down syndrome participants, a finding not previously documented with such precision.
This neuroinflammatory activity displayed a biphasic nature. Initially, microglia appear neuroprotective, attempting to mitigate damage caused by genetic and molecular disturbances. Over time, however, this protective response shifts to a pro-inflammatory state, exacerbating neuronal injury and accelerating neurodegeneration. This maladaptive immune response could be a driving force behind the earlier onset and increased severity of Alzheimer’s disease observed in the Down syndrome population.
Crucially, the study identified a significant correlation between the extent of neuroinflammation and the presence of beta-amyloid plaques, particularly pronounced in individuals over 50 years. This suggests that inflammation contributes actively to amyloid aggregation, rather than merely being a consequence of plaque formation. Such insights challenge traditional sequential models of Alzheimer’s progression and underscore inflammation’s potential as an early marker and therapeutic target.
Complementing human imaging data, the research team conducted longitudinal studies on genetically modified mice that mimic Down syndrome’s neuropathology. Over two years, these animal models enabled detailed monitoring of neuroinflammatory progression in a controlled setting. The experimental approach provided profound insights into the dynamics of microglial activation and amyloid pathology, reinforcing observations from human subjects and enhancing the translational value of the findings.
The implications for clinical practice are profound. The ability to detect and quantify neuroinflammation in vivo enables early identification of individuals at risk and real-time monitoring of disease progression. It further opens possibilities for developing anti-inflammatory therapeutics aimed at halting or slowing disease onset. Given that individuals with Down syndrome exhibit distinct Alzheimer’s disease trajectories compared to the general population, personalized treatment strategies informed by such imaging biomarkers may drastically improve outcomes.
Despite the absence of a definitive cure for Alzheimer’s, this research reinvigorates hope by spotlighting a modifiable pathological process. Targeting neuroinflammation could complement current approaches focusing on amyloid clearance, potentially yielding combination therapies with enhanced efficacy. Moreover, inclusion of Down syndrome individuals in clinical trials, facilitated by these imaging methodologies, represents a crucial step toward equitable and inclusive research practices.
From a molecular perspective, the study utilizes PET imaging agents selective for TSPO (translocator protein), a marker of activated microglia, thereby directly quantifying neuroinflammatory responses. Beta-amyloid plaque burden was concurrently assessed with radiotracers binding to amyloid fibrils, enabling a comprehensive neurochemical profile. This dual-tracer approach advances biomarker research by linking neuroimmune activation dynamics with classical pathological deposits.
The research also underlines the temporal aspect of Alzheimer’s pathogenesis in Down syndrome, emphasizing that neuroinflammation is an early event potentially preceding overt cognitive decline and plaque deposition. This challenges the paradigm that amyloid alone initiates neurodegeneration, advocating for a more integrated model incorporating immune responses as critical contributors.
By elucidating mechanisms specific to Down syndrome-associated Alzheimer’s, this work enhances our broader understanding of dementia etiology, potentially informing preventative strategies for sporadic Alzheimer’s disease. The study’s findings underscore the necessity for age- and disease-specific biomarkers and therapies, advocating for a precision medicine approach in neurodegenerative disorders.
Finally, this research epitomizes the power of multidisciplinary collaboration, combining cutting-edge nuclear medicine, genetics, animal modeling, and clinical neurology. Funded by the São Paulo Research Foundation (FAPESP), the study represents a milestone in neurodegenerative research, laying the foundation for transformative therapeutic innovations that could improve quality of life for millions globally affected by Down syndrome and Alzheimer’s disease.
Subject of Research: Neuroinflammation and amyloid deposition in brains of individuals with Down syndrome.
Article Title: Neuroinflammation and amyloid load in different age groups of individuals with Down syndrome: A PET imaging study.
News Publication Date: 7-Jul-2025.
Web References:
https://alz-journals.onlinelibrary.wiley.com/doi/full/10.1002/alz.70449
https://bv.fapesp.br/en/auxilios/102982
References:
Faria, D. de P. et al. (2025). Neuroinflammation and amyloid load in different age groups of individuals with Down syndrome: A PET imaging study. Alzheimer’s & Dementia. DOI: 10.1002/alz.70449.
Image Credits: Daniele de Paula Faria
Keywords: Amyloids, Down syndrome, Neurodegenerative diseases, Medical diagnosis, Alzheimer disease
Tags: Alzheimer’s disease development in Down syndromeamyloid precursor protein overexpressionbeta-amyloid peptides aggregationearly detection of Alzheimer’s riskearly neuroinflammation in Down syndromegenetic factors in Down syndromeimplications of neuroinflammation for Alzheimer’s preventionneurodegeneration and Down syndromeneuroinflammatory patterns in brainPET imaging in neurodegenerative researchtherapeutic interventions for Alzheimer’sUniversity of São Paulo research findings