Interdisciplinary study clarifies role of protein dynamics mediated by the amino acid methionine
Credit: Ill./©: Benedikt Goretzki, Ute Hellmich
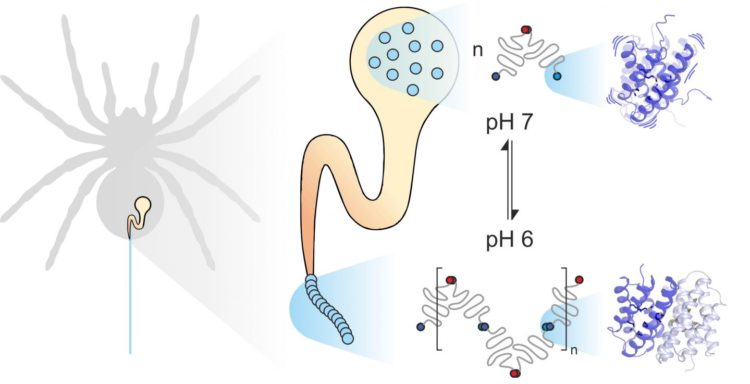
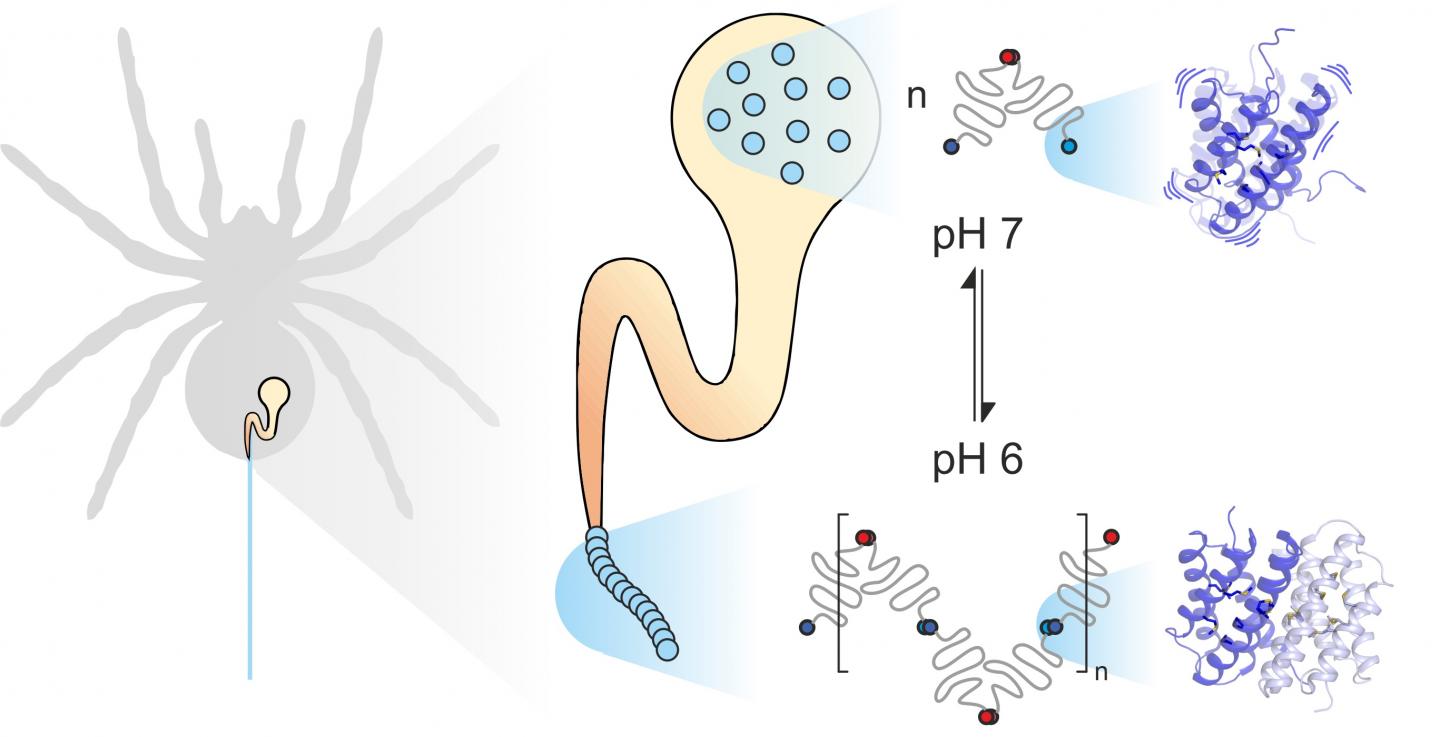
Spider silk consists of fiber-forming proteins, stored by the spider in a specialized gland. When the spider needs silk, for instance to build a web, it extrudes the silk proteins through a long duct in which they are exposed to specific mechanical and chemical influences and assembled to form silk. Spider silk proteins, like all proteins, consist of 20 elementary building blocks known as amino acids. The number and sequence of these amino acids determines the properties of individual proteins. For example, if hydrophobic amino acids such as leucine are located in the center of a protein, the result is considerable structural stability. Thus you might expect the extremely strong spider silk to contain a lot of leucine. Much to their surprise, however, scientists from the universities of Mainz and Würzburg discovered that another building block, methionine, is highly abundant in some spider silk proteins.
Methionine side chains are known to be highly flexible. “It was this abundance of methionine in the spider silk protein that made us take a closer look at its dynamics,” said Professor Ute Hellmich of Johannes Gutenberg University Mainz (JGU). “Our collaboration with the team of Dr. Hannes Neuweiler at Julius-Maximilians-Universität Würzburg (JMU) gave us access to state-of-the-art biophysical research tools.”
The Würzburg group systematically substituted the amino acid methionine in spider silk proteins with leucine and compared the folding, stability, and dynamics of the resultant protein variants with the help of photoinduced electron transfer fluorescence correlation spectroscopy (PET-FCS). Dr. Hannes Neuweiler was instrumental in the development of this technique, and his laboratory is a world leader in employing it to investigate biological systems. Professor Ute Hellmich’s team then investigated the structure and dynamics of the two protein variants using high-resolution nuclear magnetic resonance (NMR) spectroscopy. “We undertake our NMR measurements at the Center for Biomolecular Magnetic Resonance at Goethe University Frankfurt – another example of the potential generated by our cooperation in the Rhine-Main University network,” stressed Hellmich.
Methionine building blocks in spider silk proteins provide flexibility
Combining PET-FCS and NMR spectroscopy led the two research groups to the unexpected conclusion that methionine in spider silk protein increases the flexibility of the protein structure, and that this flexibility is precisely what enables the individual proteins in spider silk to interact closely. “We found that substituting methionine with leucine has no effect on the spider silk protein structure. In fact, both proteins look exactly the same. At the same time, however, the natural methionine-containing protein binds much more strongly to other spider silk proteins. The leucine-containing protein we synthesized in the lab largely loses this ability to form such stable links,” pointed out Benedikt Goretzki, a doctoral candidate in Hellmich’s team and one of the two principal authors of the study published in Nature Communications. “We were really amazed as this shows that it is not just the shape of a protein that determines how it functions but also, to a considerable degree, its flexibility.”
“Methionine not only makes the protein more dynamic, it also improves its functionality. In effect, it enables two proteins to specifically interlink with each other, which would otherwise be impossible, even if they had the same structure,” clarified Julia Heiby, doctoral candidate in Neuweiler’s group and the other principal author of the study.
“Form follows function” is a rule of thumb in structural biology. In other words, what a protein does usually can be deduced from its three-dimensional structure. “It is impressive how nature can also influence the function of proteins by precisely adapting their dynamics,” added the Mainz-based biochemist Professor Ute Hellmich.
On the basis of these findings, it may now be possible to selectively modify the properties of spider silk proteins, for example, to synthesize novel highly stable biomaterials. In addition, the two groups are also hoping to provide general insights into the relevance of the dynamics of proteins in relation to their biological functions. “Protein dynamics are important in all aspects of life,” concluded Hellmich. “This is true for both spiders and humans.”
###
Media Contact
Dr. Ute Hellmich
[email protected]
49-613-139-26182
Original Source
http://www.
Related Journal Article
http://dx.