New study using super resolution technology gives new insight into a poorly understood area of DNA replication
Credit: University of Technology Sydney
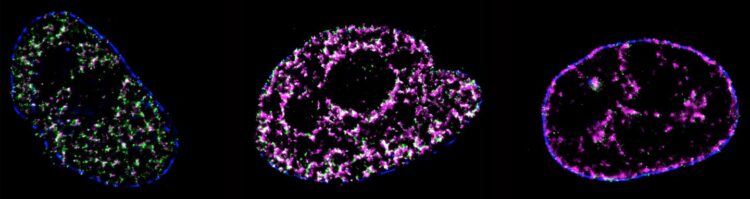
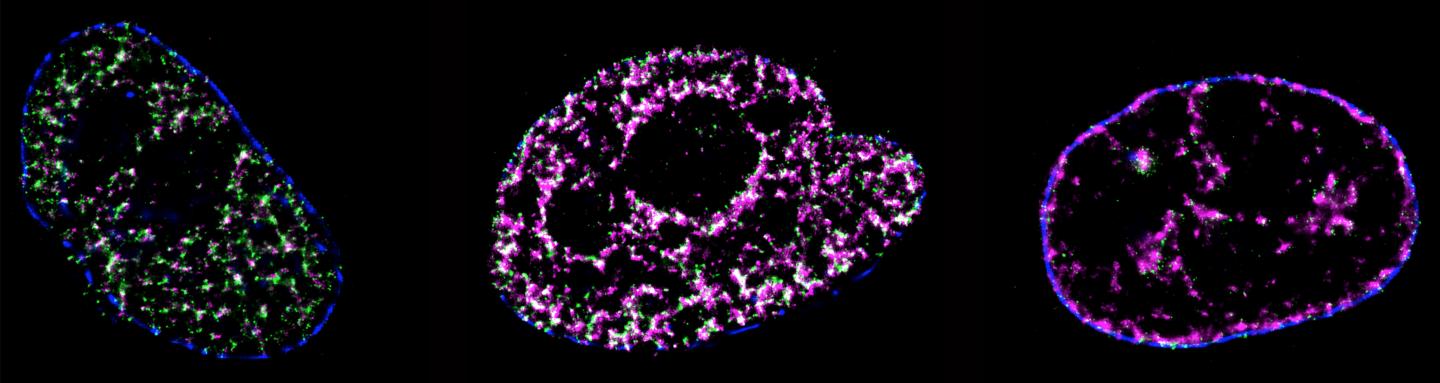
DNA replication is a process of critical importance to the cell, and must be coordinated precisely to ensure that genomic information is duplicated once and only once during each cell cycle. Using super-resolution technology a University of Technology Sydney led team has directly visualised the process of DNA replication in single human cells.
This is the first quantitative characterization to date of the spatio-temporal organisation, morphology, and in situ epigenetic signatures of individual replication foci (RFi) in single human cells at the nanoscale.
The results of the study, published in PNAS (Proceedings of the National Academy of Sciences) give new insight into a poorly understood area of DNA replication namely how replication origin sites are chosen from thousands of possible sites.
Lead author of the study, biophysicist Dr Peter Su from UTS Institute of Biomedical Materials and Devices (IBMD), explains that it’s known DNA replication is initiated at numerous sites along the chromosomes.
“These are known as replication origins, which are clustered into thousands of replication domains (RDs) across the genome, which in turn cluster within the cell nucleus as RFi ” he says.
“Such organization is critically important for the cell but how replication origins are chosen within individual RDs remains poorly understood, and it is unclear whether the origins are activated randomly or preferentially near certain chromatin features,” he says.
Chromatin helps package DNA material together so it can fit efficiently within the nucleus of a cell a, thus protecting the DNA from damage.
The collaboration with scientists from Peking University and National University of Singapore revealed a distinct pattern of replication propagation dynamics that reverses directionality across S-phase of the cell cycle, and is diminished upon knockdown of CTCF, a key regulator of 3D genome architecture.
The researchers say that together with simulation and bioinformatic analyses, these findings point to a model in which replication is preferentially activated on CTCF-organized looped chromatin structures, and suggest a non-random selection mechanism for replication activation at the sub-RD level.
Dr. Su said: “Our findings shed critical insights into the role local epigenetic environment plays in coordinating DNA replication across the genome, and could have wide-ranging implications for our understanding of how multi-scale chromatin architecture controls the organization and dynamics of diverse intranuclear processes in space and time.”
###
Media Contact
Marea Martlew
[email protected]
Related Journal Article
http://dx.