The idea of growing replacement tissue to repair an organ, or to swap it out for an entirely new one, is rapidly transitioning from science fiction to fact. Tissue engineering techniques are improving in their ability to generate three-dimensional masses of cells and provide them with vascular systems for keeping them alive, but a more mathematically rigorous approach for designing these tissues is still needed.
Researchers at the University of Pennsylvania, Brown University and ETH Zurich have now used a series of experiments to develop a dynamic model of the stresses that stretch growing tissue. This model is the first to take into account the complicated feedback effects of cells’ molecular motors, which can respond to external stress by pulling harder on their environment, eventually tearing the tissue apart.
The study was led by Vivek Shenoy, professor in the School of Engineering and Applied Science’s Department of Materials Science and Engineering, Christopher Chen, then a professor in the Department of Bioengineering, and Jeffrey Morgan of Brown’s Center for Biomedical Engineering. Hailong Wang, a member of Shenoy’s lab, was the study’s lead author; Thomas Boudou of Chen’s lab, Alexander Svoronos and Jacquelyn Youssef Schell of Brown, and Mahmut Selman Sakard of ETH Zurich also contributed to the research.
It was published in the Proceedings of The National Academy of Sciences.
“An important theme in regenerative medicine is that tissues and cells can alter their properties and behaviors, such as whether or not they want to divide, based on biochemical cues as well as mechanical cues,” Shenoy said. “Our aim was to generate a more comprehensive understanding of some of those cues, so we can build more accurate models to predict how tissues will behave when we grow them in the lab.”
The cues the researchers were particularly interested in are the ones related to how tissues pull on themselves and their surrounding environment. Much of this behavior is due to myosin activity. Myosin is a molecular motor protein that helps muscles contract, but it is present in most types of cells, as it helps them to divide and anchor themselves to one another.
To flesh out their understanding of myosin’s role in tissue stability, the researchers performed a series of physical experiments, growing heart tissue samples under various mechanical constraints while chemically altering their myosin activation.
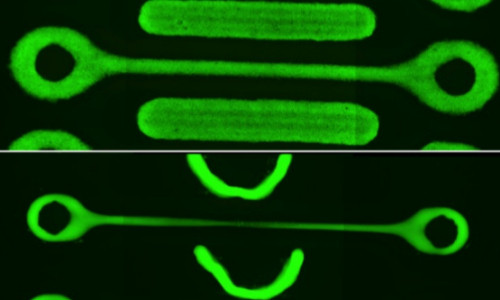
The researchers used rapid prototyping to build “dogbone” shaped environments for the tissues to grow in. The shape, two ring-shaped wells connected by a narrow bridge, was ideal for testing mechanical properties of the cells because it forced them to stretch instead of contracting in on themselves.
The researchers also fluorescently labeled the cells so they could measure the degree to which they were stretching. They saw cells in the middle of the “bone” grow up to thirty times longer than normal, a factor that led to the tissue’s ultimate demise.
“Over the course of 30 hours or so, the cells pull on one another until the middle of the bone breaks. A similar process happens in each of the rings afterwards as well,” Shenoy said. “You can’t really hold the tissue and have it stable in this shape. It will find a way to pull, due to the myosin in the cells, and this leads to a major morphological instability.”
The researchers knew myosin was responsible for this instability because when they treated the tissue samples with a drug that inhibits the protein’s activation, the tissues didn’t break. The protein transforming growth factor beta, a protein that is a factor in scarring and tumor progression, had the opposite effect on myosin, causing the tissues to break faster.
The researchers used a different experimental set-up to test another factor that could impact myosin activity: how strongly the cells are able to pull on their environment. In this set of experiments, they strung the growing heart tissues between two flexible posts. They were able to measure the strength of the tissue’s pull by observing how much the posts moved.
As with the previous set of experiments, naturally growing tissues would pull themselves apart in a matter of hours. But by altering the stiffness of the posts, or by increasing the amount of collagen, a key component of the extracellular matrix that holds the tissue together and determines how hard it can pull, the researchers found that they could make the tissues last longer.
“In the control set-up with the amount of collagen you would normally see in the heart, we see this bridge of tissue pull itself apart by day three,” Shenoy said, ”If you increase the collagen, or make the posts less stiff, you see that the tissues becomes more stable.”
”The myosin in the cells are pulling on actin filaments attached to the inside of the cells’ membranes, but if you anchor the cells, the contraction rate will decrease, eventually going to zero. That’s isometric tension at work,” he said.
With these factors quantified, Shenoy and his colleagues were able to build a more comprehensive model of mechanical failure when cells pull on one another. Their model was able to solve a long-standing conundrum found in previous models: all of the molecular components of a cell suggest that it should act like a “strain-hardening” material, something that gets tougher when pulled upon.
“These previous models were passive, they looked at all of the components of the cells but didn’t take into account the dynamic role that myosin plays,” Shenoy said. “Now that we have these three mechanical factors in our model, the cell-to-cell contacts, the role the extracellular matrix plays, and now the active myosin element, we can truly understand the sources of instability.”
Their model enabled the researchers to generate a phase diagram for growing tissue, showing where the interplay between the three factors would produce a tissue that wouldn’t stretch itself apart.
“This model will be helpful in figuring out the kind of geometry to grow artificial tissues in, such that they will redistribute the stress from these factors so they are stable,” Shenoy said. “A honeycomb, for example, can be stable and have the advantage of having channels where you can diffuse nutrients to all the cells, like a circulatory system. Our model would help you figure out the ideal spacing and diameters for the holes.”
This new level of understanding could also be applied to morphogenesis, the stage of embryonic development in which cells start differentiating into different body parts. In morphogenesis, formerly uniform masses of cells start generating highly localized differences, including in the amount of strain the cells in those locations put on one another. By understanding these cues, tissue engineers could better predict, or even manipulate, what types of tissues those undifferentiated cells will transform into.
Story Source:
The above story is based on materials provided by UPENN.