A team of scientists led by Cold Spring Harbor Laboratory (CSHL) have discovered that disulfiram, an FDA-approved drug, prevents the immune system from producing toxic webs known as neutrophil extracellular traps (NETs). Many scientists suspect NETs help drive the development of acute respiratory distress syndrome (ARDS) in patients with severe COVID-19 and other life-threatening lung injuries.
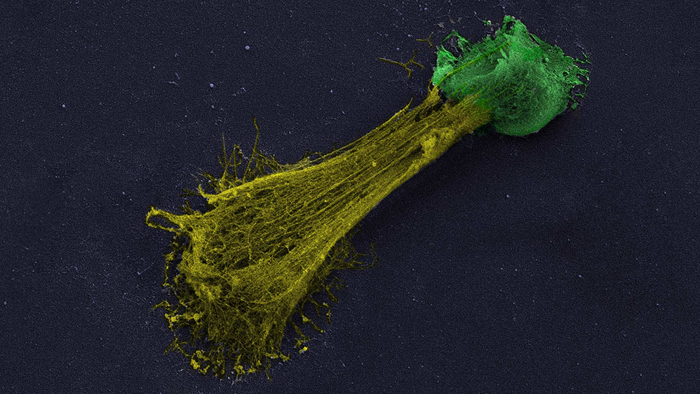
Credit: Jose M. Adrover/Egeblad lab/CSHL, 2022
A team of scientists led by Cold Spring Harbor Laboratory (CSHL) have discovered that disulfiram, an FDA-approved drug, prevents the immune system from producing toxic webs known as neutrophil extracellular traps (NETs). Many scientists suspect NETs help drive the development of acute respiratory distress syndrome (ARDS) in patients with severe COVID-19 and other life-threatening lung injuries.
Jose M. Adrover, a postdoctoral fellow in CSHL Professor Mikala Egeblad’s lab, explains that NETs are usually released during infections when immune cells, called neutrophils, confront a threat that is too large for the tiny cells to battle directly. To extend their reach, neutrophils spew a sticky web of DNA and toxins, which indiscriminately poisons pathogens and the body’s own cells. “They will damage everything, all around,” Adrover says.
Because NETs can be so destructive, researchers in Egeblad’s lab have been searching for ways to block their formation. Disulfiram, which has been used since the 1950s as a treatment for alcohol use disorders, was a promising candidate. “Disulfiram interferes with gasdermin D, a molecule needed to produce NETs”, says Juliane Daßler-Plenker, a postdoctoral fellow in Egeblad’s lab.
The team that included Weill Cornell Medicine (WCM), and Icahn School of Medicine at Mount Sinai (Mt. Sinai) investigated disulfiram’s effects on NET production. They found that the drug prevents neutrophils isolated from blood from generating NETs. Then, they gave disulfiram to mice with acute lung injuries: “By computerized tomography [CT scan], we saw a stark reduction of edema [fluid] in the lungs, and the drug dramatically improved survival,” says Scott Lyons, head of Animal Imaging at CSHL. Robert Schwartz’s team (WCM) and Benjamin tenOever’s team (Mt. Sinai) tested disulfiram in hamsters infected with the SARS-CoV-2 virus; NET production was blocked and lung injury was reduced.
Disulfiram is the first FDA-approved drug that can block NET formation. In this study, Egeblad’s team dissects the drug’s ability to block NETs and change immune signaling in a way that may be beneficial for treating severe COVID-19. Their results are reported in JCI Insight.
Clinical trials investigating disulfiram’s use in patients with symptomatic COVID-19 are underway, and while CSHL scientists are not involved in those studies, Egeblad says, “Our findings provide a reason to hope that disulfiram may be a useful treatment. We will continue exploring the drug’s potential in a variety of conditions. Just as important, we now have a tool to help us study the complex roles of NETs in lung injury, cancer, and other diseases that involve NETs.”
Journal
JCI Insight
DOI
10.1172/jci.insight.157342
Article Title
“Disulfiram inhibits neutrophil extracellular trap formation protecting rodents from acute lung injury and SARS-CoV-2 infection”
Article Publication Date
8-Feb-2022




