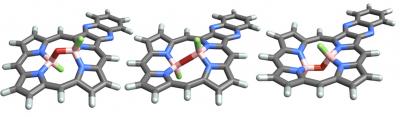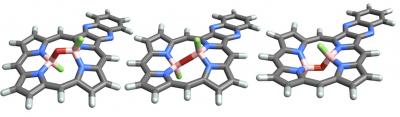
Credit: University of Sydney
Scientists have found a new way of joining groups of atoms together into shape-changing molecules – opening up the possibility of a new area of chemistry and the development of countless new drugs, microelectronics and materials with novel characteristics.
Discoveries of new ways to make isomers – molecules made of the same atoms connected together differently – were last reported in 1961 and before then in 1914.
The discovery of another form of isomerism means a whole new range of materials could be prepared, either with the same functions as existing one, or with properties currently out of reach.
As well as new types of drugs, other potential real-world applications include new materials that can be manipulated to be "switched on or off", polymers with special performance characteristics and possibly new molecular information storage devices.
The findings are published today in Nature Chemistry.
The paper was led by University of Sydney PhD candidate Peter Canfield, working closely with his supervisors Professor Maxwell Crossley, a synthetic organic chemist at the University of Sydney, and Professor Jeffrey Reimers, a theoretical chemist from The University of Technology Sydney (UTS) and Shanghai University.
Mr Canfield, who is undertaking his PhD in Sydney's School of Chemistry, said he was excited by the possibilities of what might be achieved stemming from the findings and the team was pursuing commercial applications.
"Proof-of-principle and prototype demonstration could be as early as 30 months or less," Mr Canfield said.
Professor Crossley said: "When you have a new discovery like this, there will be important applications but exactly how and when is not always anticipated at the time."
Professor Reimers said: "Our team's advance sits at the same level of understanding as Louis Pasteur's discovery of chirality – a central feature of most modern molecular science."
Professor Reimers said the mathematics of geometry describes the fundamental ways in which atoms could be combined and hence all possible types of isomers.
"When we looked at this, we noticed a fundamental form which had never been made before," he said.
The team used nanoscale porphyrin scaffolds developed by Professor Crossley to "host" boron "guest" molecules, resulting in isolable compounds – molecules stable in a bottle at room temperature.
Professor Crossley explains: "Porphyrins are very widely used by nature and by designers to grab and transport molecules and energy – we demonstrate new ways of binding guests to make this happen."
State-of-the-art spectroscopy and computational modelling at the National Computational Infrastructure in collaboration with researchers at the Australian National University gave the team confirmation that what they'd synthesised was new.
Professor Reimers concludes: "Now that it is known that isolable isomers can be made in this way, the possibilities of what chemists could make are endless."
###
Media Contact
Vivienne Reiner
[email protected]
61-293-512-390
@SydneyUni_Media
http://www.usyd.edu.au/
Related Journal Article
http://dx.doi.org/10.1038/s41557-018-0043-6