Blocking function of a blood-clotting protein prevented bone loss from periodontal (gum) disease in mice, according to research led by scientists at the National Institute of Dental and Craniofacial Research (NIDCR), part of the National Institutes of Health. Drawing on animal and human data, the researchers found that buildup of the protein, called fibrin, triggers an overactive immune response that damages the gums and underlying bone. The study, which was published in Science, suggests that suppressing abnormal fibrin activity could hold promise for preventing or treating periodontal disease, as well as other inflammatory disorders marked by fibrin buildup, including arthritis and multiple sclerosis.
Credit: Lakmali Silva, NIDCR
Blocking function of a blood-clotting protein prevented bone loss from periodontal (gum) disease in mice, according to research led by scientists at the National Institute of Dental and Craniofacial Research (NIDCR), part of the National Institutes of Health. Drawing on animal and human data, the researchers found that buildup of the protein, called fibrin, triggers an overactive immune response that damages the gums and underlying bone. The study, which was published in Science, suggests that suppressing abnormal fibrin activity could hold promise for preventing or treating periodontal disease, as well as other inflammatory disorders marked by fibrin buildup, including arthritis and multiple sclerosis.
Periodontal disease affects nearly half of Americans over age 30, and 70% of those 65 and older. It is a bacterial infection of the tissues supporting the teeth. In its early stages, periodontal disease causes redness and swelling (inflammation) of the gums. In advanced stages, called periodontitis, the underlying bone becomes damaged, leading to tooth loss. While scientists have known that periodontitis is driven in part by an exaggerated immune cell response, until now, it was unclear what triggered the response, and how it caused tissue and bone damage.
”Severe periodontal disease can lead to tooth loss and remains a barrier to productivity and quality of life for far too many Americans, especially those lacking adequate access to dental care,” said NIDCR Director Rena D’Souza, D.D.S., Ph.D. “By providing the most comprehensive picture yet of the underlying mechanisms of periodontal disease, this study brings us closer to more effective methods for prevention and treatment.”
At sites of injury or inflammation, fibrin normally plays a protective role, helping to form blood clots and activating immune cells to fight infection. But too much fibrin has been linked with health problems, including a rare form of periodontitis due to a condition called plasminogen (PLG) deficiency. In affected people, mutations in the PLG gene lead to fibrin buildup and disease at various body sites, including the mouth.
To explore the connection between abnormal fibrin buildup and periodontitis, the scientists, led by NIDCR investigators Niki Moutsopoulos, D.D.S., Ph.D., and Thomas Bugge, Ph.D., studied PLG deficiency in mice and analyzed human genetic data.
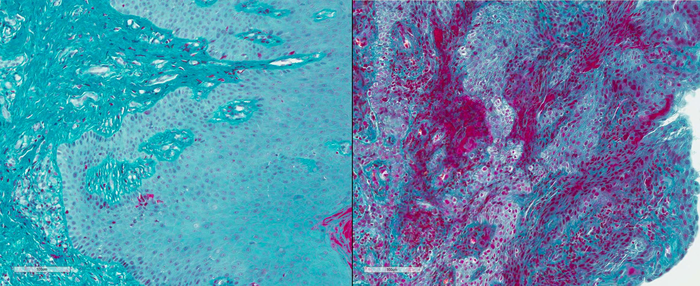
Like humans with the condition, PLG-deficient mice developed periodontitis, including periodontal bone loss and elevated levels of fibrin in the gums. The mice’s gums were crowded with immune cells called neutrophils, which are also found at high levels in common forms of periodontitis.
Neutrophils typically defend the oral cavity from harmful microbes. But an excessive neutrophil response is thought to cause tissue damage.
To find out if fibrin was driving this overactive response, the researchers impaired its ability to interact with (bind to) protein receptors on neutrophils. The weakened binding between fibrin and neutrophils completely prevented periodontal bone loss in PLG-deficient mice. Strikingly, it also reduced bone loss in normal mice with a common, age-related form of periodontitis, suggesting that similar mechanisms were at play in both forms of the disease.
“This study suggests that fibrin can cause neutrophil immunity to shift from protective to damaging in certain circumstances,” said Moutsopoulos, who credited postdoctoral fellow and study first author Lakmali Silva, Ph.D., for her research that led to the findings. “This fibrin-neutrophil engagement may be a driver of periodontitis.”
A genetic analysis of over 1,000 people seemed to support the animal findings. Even in the absence of PLG deficiency, variations in the PLG gene were linked to an increased risk of severe periodontitis, consistent with the idea that similar processes contribute to rare and common forms of the disease.
Taken together, the study suggests that excessive buildup of fibrin in the gums—whether due to changes in genes like PLG, chronic inflammation from a bacterial infection, or some combination of the two—triggers an elevated and ultimately harmful neutrophil response that causes periodontal disease.
The results are also in line with findings from other research teams, which have found that elevated fibrin may contribute to other inflammatory and autoimmune diseases such as arthritis and multiple sclerosis, and that interfering with fibrin activity could help treat these conditions.
“Our data support the idea that targeting the fibrin-neutrophil interaction could be a promising treatment avenue to explore in both rare and common forms of periodontitis,” added Silva.
This research was supported by the NIDCR Division of Intramural Research. Support also came from the intramural programs of the National Institute on Deafness and Other Communication and the National Institute of Allergy and Infectious Diseases, the extramural programs of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Fund for Scientific and Technological Development, Chile.
About the National Institute of Dental and Craniofacial Research: NIDCR is the nation’s leading funder of research on oral, dental, and craniofacial health.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases.
Reference: Silva LM, et al. Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier. Science. December 23, 2021. DOI: 10.1126/science.abl5450
Journal
Science
DOI
10.1126/science.abl5450
Method of Research
Experimental study
Subject of Research
Animals
Article Title
Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier
Article Publication Date
24-Dec-2021
COI Statement
Author C.J.K. is a director and shareholder of NanoVation Therapeutics Inc., which is commercializing RNA-based therapies. C.J.K., L.J.J., and J.L. are inventors on pending intellectual property related to RNA-based therapies. The other authors declare that they have no competing interests.




