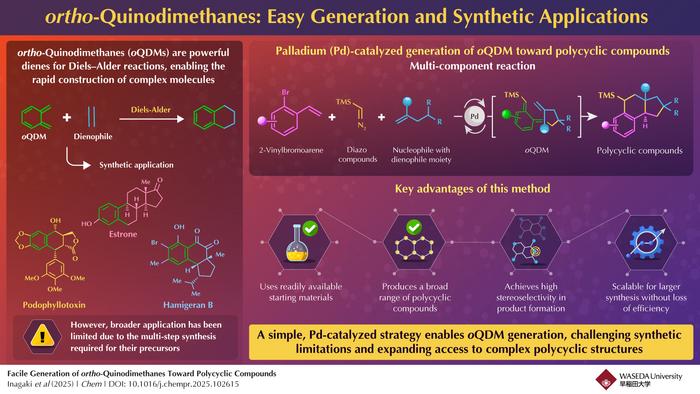
In the expansive domain of organic chemistry, constructing intricate molecular architectures with precision and efficiency remains a persistent challenge. Among the suite of synthetic tools, the Diels–Alder reaction has long been heralded as a keystone transformation, renowned for its capacity to forge complex polycyclic frameworks with remarkable regio- and stereoselectivity. Central to the success of many Diels–Alder-based synthetic routes is the deployment of highly reactive dienes capable of engaging in cycloaddition under mild conditions. A particularly intriguing class of these dienes is the elusive ortho-quinodimethane (oQDM) intermediate, which, owing to its reactive nature, serves as a pivotal building block for constructing fused ring systems prevalent in natural products and biologically active compounds.
Despite its synthetic allure, access to ortho-quinodimethanes has traditionally been impeded by the necessity for harsh reaction conditions and the laborious synthesis of precursors, often limiting its widespread application in total synthesis and medicinal chemistry. For over seventy years, efforts to tame and exploit oQDM intermediates have been restrained by their fleeting existence and propensity for undesirable side reactions. This inherent instability has posed a formidable barrier, hindering the development of straightforward, generalizable methods for its generation and subsequent polycyclic compound construction.
Addressing this longstanding synthetic conundrum, an innovative research team led by Professor Junichiro Yamaguchi from Waseda University, in collaboration with Dr. Kei Muto at the Institute of Transformative Bio-Molecules, Nagoya University, has unveiled a groundbreaking palladium-catalyzed multicomponent reaction capable of generating oQDM species in situ. This transformative methodology elegantly orchestrates the union of 2-vinylbromoarenes, diazo compounds, and carbon nucleophiles that contain dienophile moieties, thereby facilitating a streamlined access route to complex polycyclic structures without the necessity of isolating or stabilizing the highly reactive intermediate.
.adsslot_fot6pRLdmh{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_fot6pRLdmh{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_fot6pRLdmh{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
The heart of this approach lies in the catalytic generation of a benzyl–palladium intermediate, a reactive species that acts as a launchpad for carbon–carbon bond formation. Harnessing palladium’s versatile coordination chemistry and catalytic prowess, the researchers have engineered a reaction environment conducive to the controlled release and subsequent trapping of ortho-quinodimethane intermediates. This method circumvents the traditional pitfalls associated with oQDM generation, markedly reducing reaction steps and eliminating the need for harsh reagents or extreme temperatures that have historically constrained synthetic chemists.
Fundamentally inspired by nature’s own efficiency in constructing complex molecular motifs from simple building blocks, the new protocol exemplifies biomimetic principles in synthetic design. The research team drew parallels between enzymatic catalysis, which meticulously guides reactive intermediates through intricate biosynthetic pathways, and their palladium-catalyzed system that modulates oQDM reactivity with precision. This bioinspired approach not only broadens the synthetic utility of ortho-quinodimethanes but also exemplifies a paradigm shift towards more sustainable and practical synthetic organic chemistry.
The scope of this methodology was rigorously demonstrated through the synthesis of a diverse array of polycyclic compounds, including the natural product equilenin, a steroid hormone analog relevant to biological research and pharmaceutical development. By successfully assembling such complex frameworks with high fidelity, the team showcased the potential of their platform to impact the synthesis of bioactive molecules and structurally sophisticated natural products. Notably, the presence of a vinyl substituent in the final polycyclic products opens further avenues for functionalization, enabling chemists to build libraries of compounds suited for drug discovery and material science applications.
A pivotal advantage of this palladium-catalyzed protocol is the significant reduction in synthetic complexity and reaction harshness compared to classical methods. Traditional oQDM generation and utilization often required thermally induced or photochemical protocols with narrow substrate tolerance. In contrast, Yamaguchi’s team demonstrated that their multicomponent reaction proceeds under milder conditions, utilizing commercially available reagents and ambient operational parameters. These attributes collectively empower synthetic chemists to innovate freely without the constraints imposed by earlier synthetic inconveniences.
Given the reactivity and versatility of the generated polycyclic scaffolds, the methodology bears immense promise for accelerating pharmaceutical discovery efforts. Access to such molecular frameworks, which include motifs common in hormone-based therapeutics and anticancer agents, facilitates rapid analog synthesis and structure-activity relationship studies. Moreover, the ability to construct compound libraries efficiently supports high-throughput screening pipelines, advancing the search for novel therapeutic agents and functional materials with enhanced biological or physicochemical properties.
Professor Yamaguchi emphasizes that this research not only responds to a classic synthetic challenge but also introduces a catalyst-controlled framework that could redefine how chemists manipulate transient intermediates. The strategic inclusion of diazo species and carbon nucleophiles containing dienophile groups is a masterstroke that allows for the synchronous orchestration of multiple reactive sites, embodying a multidimensional synthetic strategy. Such synergy between different reactive components within a catalytic cycle exemplifies a forward-looking approach to reaction design in organic synthesis.
Beyond its immediate synthetic goals, this work highlights the growing trend of integrating organometallic catalysis with intricate reaction cascades to achieve complexity from simplicity. The benzyl–Pd intermediate, once viewed merely as a transient species, emerges here as a crucial node enabling seamless carbon–carbon bond formation en route to architecturally rich molecules. This study thus enriches our understanding of palladium’s catalytic versatility and inspires further exploration of dynamic reaction networks leveraging metal-catalyzed intermediate generation and capture.
In an era where efficiency, sustainability, and innovation guide synthetic chemistry, the advancement reported by Yamaguchi and collaborators sets a new benchmark. Their method not only revives interest in ortho-quinodimethanes as valuable synthetic intermediates but also illustrates how modern catalysis can transform conceptual challenges into practical, accessible solutions. Such progress resonates beyond academia, promising implications for drug development, agrochemicals, and advanced material synthesis.
Looking ahead, the research team’s approach paves the way for expanding reaction scope and functional group tolerance, potentially enabling enantioselective variants or scalability required for industrial application. Further exploration of substrate diversity and mechanistic studies could unlock additional reactivity modes, extending the utility of this elegant catalytic platform. As synthetic chemists continue to aspire toward precision and complexity, the facile generation of oQDM intermediates exemplified here will undoubtedly serve as a cornerstone in the ongoing quest to master molecular construction.
Subject of Research:
Article Title: Facile Generation of ortho-Quinodimethanes Toward Polycyclic Compounds
News Publication Date: 2-Jun-2025
Web References: https://doi.org/10.1016/j.chempr.2025.102615
References: Inagaki, K., Onozawa, Y., Fukuhara, Y., Yokogawa, D., Muto, K., & Yamaguchi, J. (2025). Facile Generation of ortho-Quinodimethanes Toward Polycyclic Compounds. Chem. https://doi.org/10.1016/j.chempr.2025.102615
Image Credits: Professor Junichiro Yamaguchi, Waseda University
Keywords
Organic synthesis, Chemical synthesis, Organic chemistry, Drug discovery, Medicinal chemistry, Materials science, Catalysis, Organic compounds, Molecular chemistry, Organometallic chemistry
Tags: building blocks for natural productschallenges in total synthesisDiels–Alder reaction applicationsDirect synthesis of complex moleculesinnovative organic synthesis techniquesmedicinal chemistry advancementsnovel methods for oQDM generationortho-quinodimethanes in organic chemistryovercoming synthetic limitationspolycyclic frameworks synthesisreactive diene intermediatesregio- and stereoselectivity in synthesis