Urinary and bowel dysfunctions, often relegated to the shadows of medical concern, impose a significant burden on millions worldwide, disrupting daily life and eroding quality of life despite their invisible nature. These disorders encompass a spectrum of symptoms including frequent, urgent urination, difficulty initiating voiding, and impaired bowel movements. Although common, particularly among the elderly, their impact transcends mere inconvenience, often signaling underlying neurological pathologies that necessitate prompt identification and sophisticated management strategies. Unfortunately, stigma and a lack of awareness frequently delay diagnosis, exacerbating patient suffering and complicating therapeutic interventions.
In a groundbreaking advancement poised to redefine the management of urinary disorders, a research collective from South Korea’s Pohang University of Science & Technology (POSTECH) and Hanyang University has unveiled a cutting-edge neuromodulation control mechanism. This novel technique harnesses real-time bioelectrical feedback derived from the Evoked Compound Action Potential (ECAP) to finely calibrate tibial nerve stimulation, a promising treatment modality for overactive bladder syndrome (OAB). Their pioneering work, recently published in Nature Communications on May 2, 2025, deepens our understanding of peripheral nerve modulation and presents a transformative leap towards precision medicine in neuro-urology.
The clinical condition targeted by this innovation, overactive bladder syndrome, is characterized by involuntary bladder contractions resulting in sudden urges to urinate, increased frequency of urination, and, in some cases, urinary incontinence. The condition presents a broad clinical spectrum, extending from mild discomfort to debilitating dysfunction that severely restricts autonomy, especially in aging populations. Current therapies range from pharmacological agents to lifestyle adjustments and invasive surgical procedures, yet many patients remain refractory or experience adverse effects, underscoring the imperative for improved interventions.
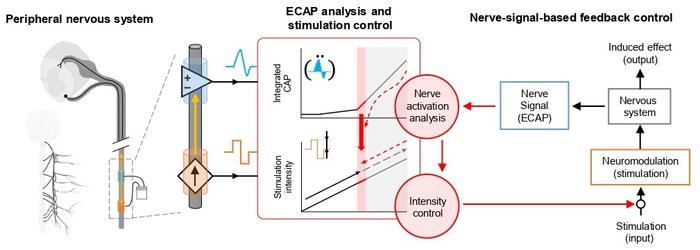
Neuromodulation has surfaced as a compelling therapeutic avenue, utilizing electrical impulses delivered to peripheral nerves controlling bladder function to modulate aberrant signals. Traditionally, clinicians have relied on empirical techniques, adjusting stimulation based on observable physical responses or patient-reported outcomes. This approach inherently introduces variability influenced by subjective interpretation, clinician experience, and patient compliance, limiting the consistency and effectiveness of outcomes. Addressing these limitations demands a paradigm shift towards objective, real-time feedback systems that can dynamically adapt stimulation parameters.
Central to this breakthrough is the application of the Evoked Compound Action Potential—a measurement of the collective electrical response of nerve fibers following stimulation. By directly capturing ECAP signals emanating from the tibial nerve, the system functions analogously to a conductor adjusting an orchestra’s tempo by attentively perceiving subtle acoustic cues. This bioelectrical feedback loop enables clinicians to precisely tailor stimulation intensities and frequencies, synchronized with individual neural responses, thereby moving away from one-size-fits-all regimens to truly personalized neuromodulation therapies.
The innovation extends beyond conceptual excellence; the team engineered a compact, implantable device equipped with specialized electrodes designed for chronic application in animal models exhibiting OAB symptoms. This miniaturized platform not only delivers precise electrical stimuli but concurrently records ECAPs in real-time, creating a closed-loop system that automatically optimizes stimulation parameters. Animal trials revealed pronounced amelioration of OAB symptoms, validating the efficacy of objective feedback integration compared to conventional methodologies reliant on indirect clinical signs such as muscle twitches.
What sets this technology apart is its capacity for autonomous adaptation. In contrast to previous neuromodulation techniques requiring continuous clinician involvement and subjective titration, the ECAP feedback system autonomously calibrates stimulation, responding instantaneously to neural state fluctuations. This dynamic modulation ensures maintenance of therapeutic thresholds while minimizing overstimulation risks, ultimately fostering improved safety profiles and enhancing patient comfort. Such precision heralds a new era in neurotherapeutics where device intelligence complements clinical acumen.
The implications of this advancement are profound and extend well beyond urinary disorders. As Professor Sung-Min Park of POSTECH, a leading figure in this research, elucidated, “The ability to non-invasively measure ECAP signals from peripheral nerves unlocks vast potential across neurological disease management.” Drawing from his distinguished tenure at Medtronic, where he contributed to MRI-compatible implantable pacemaker development, Professor Park emphasizes the translational applicability of this technology. Its adaptability suggests promising integration into diverse clinical domains including gastrointestinal, cardiovascular, and chronic pain disorders, wherein neuromodulation is rapidly gaining traction.
Simultaneously, the research addresses critical challenges in biomedical engineering, notably the miniaturization and biocompatibility of implantable devices. The design encompasses electrodes optimized for stable nerve interface and low power consumption to ensure long-term functionality. Such engineering excellence equips the system to overcome physiological hurdles like biofouling and inflammatory responses, thereby ensuring reliable chronic use. This convergence of sophisticated hardware and refined signal processing algorithms positions the device at the forefront of next-generation bioelectronic medicines.
Looking forward, this technology’s scalability and versatility portend transformative impacts on healthcare delivery frameworks. By providing clinicians with objective, quantifiable neural biomarkers, the approach promises improved diagnostic precision and personalized therapy adjustments without the need for frequent clinical visits. This could be particularly crucial for elderly or mobility-impaired patients, facilitating remote monitoring and telemedicine integration. Furthermore, the closed-loop stimulation paradigm aligns with burgeoning trends in artificial intelligence-assisted medical devices, fostering synergistic opportunities for automated disease management.
Funded through significant backing by the National Research Foundation of Korea and multiple government programs aimed at pioneering scientific innovation, this research embodies a strategic investment into future healthcare solutions. It represents a confluence of interdisciplinary expertise spanning neurology, electrical engineering, and biomedical innovation, underscoring the vital role of collaborative efforts in addressing complex medical challenges. The study’s publication in a prestigious journal attests to its scientific rigor and anticipated impact within the global medical community.
In sum, the development of an ECAP-driven tibial nerve stimulation system marks a seminal achievement in neuromodulation therapy for urinary and bowel disorders. By shifting towards a feedback-informed, personalized approach, this research dismantles longstanding barriers of variability and inefficacy in treatment. It promises not only symptom relief but also a restoration of independence and dignity for patients grappling with these often-overlooked conditions. As the field of bioelectronic medicine evolves, such innovations will undoubtedly pave the path toward more intelligent, adaptive, and patient-centered interventions.
Subject of Research: Neuromodulation therapy for urinary disorders using real-time Evoked Compound Action Potential feedback.
Article Title: Precise control of tibial nerve stimulation for bladder regulation via evoked compound action potential feedback mechanisms
News Publication Date: 2-May-2025
Web References: https://doi.org/10.1038/s41467-025-59436-4
Image Credits: POSTECH
Keywords: Health and medicine; Nervous system; Peripheral nervous system; Life sciences; Anatomy; Excretory system; Bioengineering; Medical technology; Regenerative medicine; Biotechnology; Biomedical engineering; Autonomic nervous system
Tags: bioelectrical feedback in neuromodulationdirect nerve monitoringelderly urinary disordersEvoked Compound Action Potentialgroundbreaking research in urinary treatmentneurological pathologies in urinary issuesoveractive bladder syndrome managementpersonalized tibial nerve stimulationprecision medicine in neuro-urologystigma in urinary healthurinary and bowel dysfunctionsurinary disorder treatment