□ The research team led by Professor Minseok Kim from the Department of New Biology at DGIST (President Yang Kuk) has developed a technology that can treat Charcot-Marie-Tooth (CMT) disease, an incurable hereditary disease, with electric stimulation instead of drug therapy. The core of this technology is electric stimulation that restores the abnormal distribution of peripheral myelin protein 22 (PMP 22)[1], the cause of the disease, to normal. The research team discovered it by conducting a series of electric stimulation experiments using a CMT disease subtype 1A (CMT1A) cell model. This technology has considerable potential for the development of an electronic medicine with minimal side effects in the future.
Credit: .
□ The research team led by Professor Minseok Kim from the Department of New Biology at DGIST (President Yang Kuk) has developed a technology that can treat Charcot-Marie-Tooth (CMT) disease, an incurable hereditary disease, with electric stimulation instead of drug therapy. The core of this technology is electric stimulation that restores the abnormal distribution of peripheral myelin protein 22 (PMP 22)[1], the cause of the disease, to normal. The research team discovered it by conducting a series of electric stimulation experiments using a CMT disease subtype 1A (CMT1A) cell model. This technology has considerable potential for the development of an electronic medicine with minimal side effects in the future.
□ CMT disease causes muscle atrophy, numbness, foot deformities, paralysis, and other symptoms due to the loss of myelin sheath in peripheral nerves. It is a genetic disease that affects a large number of people, with approximately 1 in 2,500 individuals affected. However, there is currently no definitive treatment for this disease.
□ CMT1A, a subtype of CMT disease, is among the most prevalent types of peripheral nerve damage worldwide and is caused by PMP22 overexpression in Schwann cells. PMP22 overexpression in Schwann cells causes the PMP22 protein to aggregate in the cells, ultimately leading to peripheral nerve damage.
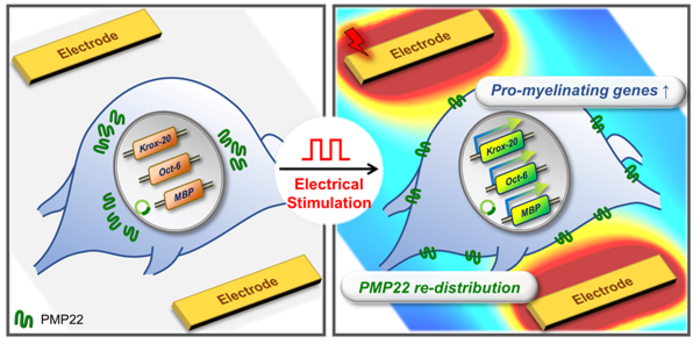
□ To address PMP22 overexpression, Professor Minseok Kim’s team developed a CMT1A cell model ‘PMP22-overexpressing schwannoma[2] cell’. This team applied a high-speed electric stimulation screening platform to the cells to identify optimal stimulation conditions for effective therapeutic outcomes. This electric stimulation eliminated the PMP22 protein aggregation around the nucleus that occurs in CMT1A disease and restored the normal distribution of the PMP22 protein towards the cell membrane, similar to that of a normal Schwann cell.
□ In addition, the research team observed an increase in the expression of genes (e.g., MBP, MAG) that play an important role in the formation of myelin sheaths constituting a part of peripheral nerves and changes in the gene expression of transcription factors (Krox-20, Oct-6, c-Jun, and Sox10) that control myelin sheath formation, leading to the induction of myelination in the CMT1A cell model.
□ Professor Minseok Kim from the Department of New Biology at DGIST stated that, “The key of this study is that it is the first demonstration that electric stimulation can restore the normal distribution of PMP22, which is a major protein in CMT1A disease.” He further expressed that, “We hope that new electric medicine technologies will soon be commercialized to address CMT disease that currently has no cure and causes suffering to millions of people worldwide.”
□ This study by Professor Minseok Kim’s research team was conducted with the support of the National Research Foundation of Korea and the results will be published in March in the prestigious bioscience journal Biosensors and Bioelectronics.
Correspondence Author E-mail : [email protected]
[1] Peripheral myelin protein 22 (PMP22): growth arrest-specific protein 3 is a protein that is encoded by the PMP22 gene in humans. PMP22 is a 22 kDa transmembrane glycoprotein composed of 160 amino acids and is primarily expressed in Schwann cells in the peripheral nervous system.
[2] Schwannoma: A tumor that develops in the nerve sheath, a tubular structure that surrounds and supports nerves.
Journal
Biosensors and Bioelectronics
DOI
10.1016/j.bios.2022.115055
Article Title
Electroceutical approach ameliorates intracellular PMP22 aggregation and promotes pro-myelinating pathways in a CMT1A in vitro model
Article Publication Date
15-Mar-2023




