Credit: Kanazawa University
[Background]
A ring-shaped molecule may possess a cavity because of its three-dimensional structure. This cavity can accommodate a guest molecule if its size (configuration) is adequate for the cavity, while it cannot if inadequate. The relationship of a ring-shaped molecule and an adequate guest molecule is like a keyhole and its key; it is called the host-guest complexation.
Host-guest complexations have been investigated mostly in solutions where both ring-shaped host molecules and guest molecules are solutes, whereas such complexations of molecules at solid/liquid or solid/gas interfaces have scarcely been investigated. Upon considering that separation and purification of substances are performed by taking solid out of liquid or gas, separation ability at solid/liquid or solid/gas interfaces should be more important.
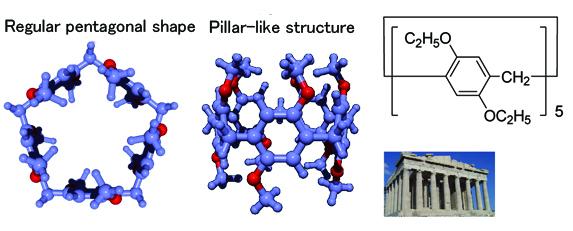
Pillar[n]arene, a ring-shaped molecule, developed by the researchers of Kanazawa University in 2008 (n indicates repetition number of the unit; see Figure 1 where n = 5) was found to show characteristics to capture adequate-sized guest molecules in solution. Pillar[n]arene could be obtained at a yield of 70% by rather easy recrystallization, and derivatives could be made by introducing various atoms or atomic groups, upon its alkoxyl group being converted into a highly reactive phenolic hydroxyl group. Since pillar[n]arene possesses a variety of superb characteristics, a number of researchers of the world have been paying much attention for possible applications.
Among its relatives, pillar[5]arene is specific since it can capture alkane gas molecules, which are difficult to be captured since such molecules consist of low-polarity groups only like carbon-carbon and carbon-hydrogen groups. This is a special characteristics/ability of pillar[5]arene to act as a host molecule. Furthermore, pillar[5]arene can be a host molecule for an n-alkane gas molecule but not for a branched or cyclic alkane gas molecule (Figure 2). This indicates that pillar[5]arene possesses special ability to distinguish configuration of molecules.
However, these phenomena could be observed only in liquids. By investigating host-guest complexation ability of pillar[5]arene at solid/gas interfaces, solid powder of pillar[5]arene was found to capture alkane gas molecules at a high efficiency by just being exposed to the gas and as well as to possess characteristics/ability to distinguish configuration of guest molecules. Furthermore, it was revealed that pillar[5]arene showed excellent property that n-alkane gas molecules once captured were not released at room temperature and under atmospheric pressure.
On the other hand, since the solid powder of pillar[5]arene is colorless, it was not possible to tell whether n-alkane gas molecules were captured or not by color, for example.
[Results]
In this study, the researchers of Kanazawa University have created a compound with special three-dimensional structure (Figure 3) upon conjugation of pillar[5]arene with benzoquinone, an electron accepting molecule, and evaluated its characteristics.
While this compound was dark-brown without guest molecules, its color turned into light-red when an n-alkane gas molecule was captured (Figure 3B). Thus, the research team was successful in detecting alkane gas molecules, which were difficult to be detected by the color change.
In addition, when exposed to branched/cyclic alkane gas molecules, the color of the compound was found to be unchanged (Figure 3C). Furthermore, when exposed to a mixture of gas molecules, i.e., a mixture of n-, cyclic and branched alkanes, the compound selectively adsorbed n-alkane molecules and changed its color. In other words, it is now possible, by the color change, to tell whether a mixture of gas molecules contains n-alkane gas molecules.
On the other hand, if exposed to gaseous methanol that has a hydroxyl group, the color changed from dark-brown to black (Figure 3A). Thus, functional groups of the guest gas molecules could be distinguished by the color.
It was found that the color change was brought about by the formation of charge-transfer complex; the backbone structure of pillar[5]arene is 1,4-dialkoxybenzene, an electron donating molecule moiety, transferring electric charge to benzoquinone.
About the color change from dark-brown to light-red (Figure 3B), the powder adsorbs n-alkane gas molecules, which brings about crystal structure transformation, which in turn induces structural change where intermolecular π-stacking interaction takes place between benzoquinone and 1,4-dialkoxybenzene. About the color change from dark-brown to black (Figure 3A), it is because methanol molecule does not induce such a structural interaction of benzoquinone and 1,4-dialkoxybenzene.
n-alkane with medium-length alkyl chain is gaseous, but the n-alkane gas molecules captured are found not to be released at room temperature and under atmospheric pressure. On the other hand, under a vacuum condition at 80°C, the captured molecules were found to be released.
By the way, when the gas molecules were released, the color changed again from light-red to dark-brown, the original color; when n-alkane gas molecules are captured once again, the color changed from dark-brown to light-red. This indicates that pillar[5]arene can tell host-guest complexation to take place by the color change and that recycling is readily possible.
[Significance]
The compound with a special three-dimensional structure developed in this study can capture alkane gas molecules of low polarity, which are generally difficult to be captured, accompanied with the color change. This host-guest complexation is highly selective, just like a key and its key-hole; n-alkane molecules in a mixture of n-, branched and cyclic alkane gas molecules can be detected by the color change where the n-alkane gas molecules only can be captured. Alkane gas molecules, having considerable vapor pressure, are utilized as a fuel, such as gasoline, for internal combustion engines. Since the combustion efficiency of n-alkane gas differs from that of branched alkane gas, the compound developed by this study is expected to serve as a sensor material for purity of gasoline.
In addition, the compound may be expected to be applied and utilized as materials to store the fuel of internal combustion engines, such as highly inflammable gasoline, in a stable manner.
Moreover, although the current study has focused on alkane gas molecules, upon considering that almost all organic compounds contain carbon-carbon and carbon-hydrogen groups, pillar[n]arene may capture a variety of gaseous organic molecules. Thus, it is also expected that pillar[n]arene may possess a high possibility for application like sensors for explosives and toxic materials.
###
Media Contact
Fujiko Imanaga
[email protected]
81-762-645-977
http://www.kanazawa-u.ac.jp/e/index.html
############
Story Source: Materials provided by Scienmag