In a groundbreaking study published in Nature Communications, researchers have unveiled new insights into the detectability and representativeness of circulating tumor DNA (ctDNA) across multiple body fluids in patients with metastatic breast cancer. This investigation marks a pivotal advance in liquid biopsy technology, a method that has transformed oncology by enabling minimally invasive tumor characterization through analysis of tumor-derived genetic material circulating in body fluids. The study’s comprehensive comparison of seven different body liquids offers a nuanced understanding of ctDNA dynamics and holds promise for revolutionizing cancer diagnostics and monitoring.
Liquid biopsy, a technique that detects tumor-specific DNA fragments shed into bodily fluids, has emerged as a powerful tool for cancer management. Unlike traditional tissue biopsies, which are invasive and limited to accessible tumor locations, liquid biopsies provide a safer and repeatable means to capture the genetic landscape of tumors. However, the heterogeneity in ctDNA abundance and integrity across different biological fluids remains a major challenge. The research led by Richard, Maetens, Van Baelen, and colleagues addresses this gap by systematically evaluating ctDNA detection rates and representativity in plasma, urine, cerebrospinal fluid, pleural effusion, ascites, saliva, and menstrual fluid obtained from metastatic breast cancer patients.
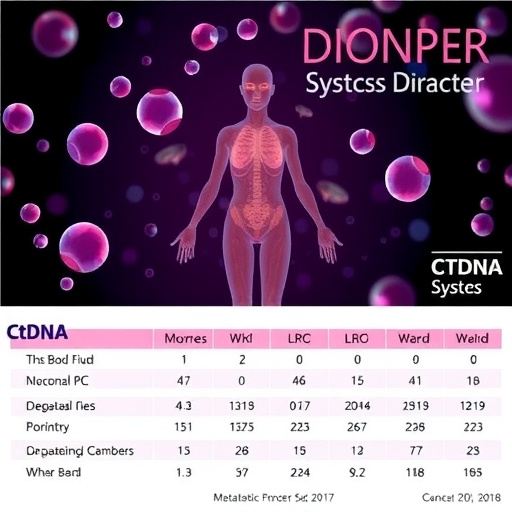
The study involved meticulous sample collection and advanced sequencing techniques to identify tumor-specific mutations within the ctDNA extracted from the seven liquids. Plasma, traditionally regarded as the gold standard for liquid biopsy, served as the reference against which other body fluids were compared. Remarkably, the findings demonstrated that although plasma remains a highly reliable source of ctDNA, several other fluids also harbor detectable and informative tumor-derived DNA. Pleural effusions and ascitic fluids, often associated with metastatic disease, showed particularly high ctDNA concentrations, reflecting their proximity to tumor sites and potential as complementary sample types for comprehensive molecular profiling.
Urine emerged as a surprisingly informative fluid despite the anatomical distance from primary tumor sites. The data revealed that urine-derived ctDNA captures a portion of the tumor mutational landscape, enabling genetic analysis when blood samples are limited or contraindicated. Cerebrospinal fluid (CSF), critical for patients with brain metastases, offered unique insights into central nervous system tumor heterogeneity and mutations that might evade detection in the bloodstream. Saliva and menstrual fluid showed lower but nonetheless meaningful ctDNA presence, underscoring the diversity of accessible biomarkers across various biological milieus.
One of the pivotal revelations of this research lies in the concept of ctDNA representativeness: how well ctDNA from different body fluids reflects the genetic heterogeneity of metastatic breast cancer lesions. The authors demonstrated that combining analyses from multiple liquid types enhanced the detection of subclonal mutations and minimized sampling bias inherent to single-fluid biopsies. This multidimensional approach may enable oncologists to better monitor tumor evolution, therapeutic resistance mechanisms, and metastatic progression in real time.
The techniques employed in this study included ultra-sensitive next-generation sequencing platforms tailored to detect low-frequency mutations, digital droplet PCR assays for variant validation, and bioinformatics pipelines designed to dissect tumor clonal architectures from ctDNA. By comparing mutational profiles and allele frequencies across fluids within the same patient, the researchers established a robust methodological framework to assess detectability thresholds and biological representativeness.
This study also posed important questions about the biological origins and trafficking pathways of ctDNA. It highlighted that different body fluids likely capture distinct fractions of the tumor burden, shaped by tumor microenvironment, vascularization, and organ-specific dissemination patterns. These biological nuances emphasize the need for fluid-specific preanalytics and analytic optimization to maximize the clinical utility of liquid biopsies in metastatic breast cancer.
Importantly, the research has immediate translational implications. Multifluid liquid biopsy strategies could enhance personalized treatment paradigms by offering more comprehensive molecular portraits without subjecting patients to repeated invasive biopsies. This is particularly valuable in metastatic breast cancer, a highly heterogeneous disease where dynamic genetic information can inform personalized therapeutic decisions, anticipate resistance, and improve prognostication.
Moreover, this multifaceted liquid biopsy approach may accelerate drug development by facilitating biomarker-driven clinical trials. Researchers can longitudinally track tumor genetic shifts using easily accessible fluids, providing real-time feedback on treatment efficacy and guiding adaptive therapeutic interventions. Such precision monitoring has the potential to reduce treatment-related toxicities and improve patient outcomes.
The findings also underscore the promise of integrating liquid biopsy into routine oncological practice. Current clinical guidelines primarily emphasize plasma ctDNA testing; however, this study advocates expansion to include body fluids such as pleural effusions, ascites, and CSF in appropriate clinical contexts. This integration will require harmonization of sample collection, processing protocols, and standardized reporting to ensure reproducibility and clinical validity.
In summary, this extensive analysis of ctDNA across seven body fluids presents a paradigm shift in how tumor genomic information can be sourced non-invasively from metastatic breast cancer patients. It enriches our understanding of tumor biology and ctDNA kinetics while charting a roadmap for refining diagnostic assays that capture the full spectrum of metastatic heterogeneity. It elevates liquid biopsy from a single-fluid diagnostic test to an integrative multi-fluid molecular surveillance platform.
Future research building on these findings will likely explore the longitudinal dynamics of ctDNA in multifluid compartments during treatment, investigate the prognostic and predictive value of multifluid ctDNA signatures, and develop machine learning models that integrate multifluid molecular data for personalized medicine. This study thus provides a foundational resource that inspires innovative clinical protocols and technological advances aimed at conquering metastatic breast cancer.
The implications of this work resonate beyond breast cancer, as similar multifluid ctDNA analyses may transform diagnostics and treatment monitoring for diverse cancers with complex metastatic patterns. By revealing the strengths and limitations of various body fluids as reservoirs of tumor DNA, the research invites a reevaluation of liquid biopsy paradigms and stimulates the oncology field toward more holistic and precise tumor monitoring approaches.
This landmark study represents a critical step toward realizing the full potential of liquid biopsies as a universal, minimally invasive tool to unravel cancer’s genetic complexity, optimize therapeutic decisions, and ultimately improve survival outcomes for patients battling metastatic breast cancer worldwide.
Subject of Research: Detectability and representativeness of circulating tumor DNA (ctDNA) in multiple body fluids from patients with metastatic breast cancer.
Article Title: ctDNA detectability and representativeness in seven body liquids from patients with metastatic breast cancer.
Article References:
Richard, F., Maetens, M., Van Baelen, K. et al. ctDNA detectability and representativeness in seven body liquids from patients with metastatic breast cancer. Nat Commun 16, 10826 (2025). https://doi.org/10.1038/s41467-025-65838-1
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41467-025-65838-1
Tags: advancements in cancer liquid biopsycancer genetic landscape characterizationcerebrospinal fluid in cancer researchcirculating tumor DNA detectioncomparison of biological fluids for ctDNActDNA analysis in body fluidsheterogeneity of ctDNA abundanceliquid biopsy technology in oncologymetastatic breast cancer diagnosticsminimally invasive cancer monitoringplasma and urine ctDNA detectiontumor-derived genetic material