Credit: LIU Xiaodi
A joint team, while exploring phase diagrams in dense H2-HD-D2 mixtures, has reported a new discovery in which they found counterintuitive effects of isotopic doping on the phase diagram of H2-HD-D2 molecular alloy.
This work was conducted by a research team at the Institute of Solid State Physics, Hefei Institutes of Physical Science collaborating with researchers from the Center for High Pressure Science & Technology Advanced Research and University of Edinburgh. It was published in PNAS on 2 June, 2020.
Molecular hydrogen forms the archetypical quantum solid. Its quantum nature is revealed by classically impossible behavior as well as by very strong isotope effects. Isotope effects between H2, D2, and HD molecules come from mass difference and the different quantum exchange effects: Fermionic H2 molecules have antisymmetric wavefunctions, while bosonic D2 molecules have symmetric wavefunctions, and HD molecules have no exchange symmetry.
To investigate how the phase diagram depends on quantum-nuclear effects, the joint team used high-pressure and low-temperature in situ Raman spectroscopy to map out the phase diagrams of H2-HD-D2 with various isotope concentrations over a wide P-T range.
When hydrogen and deuterium were mixed, they formed H2 + HD + D2 mixtures at very low pressures and room temperature.
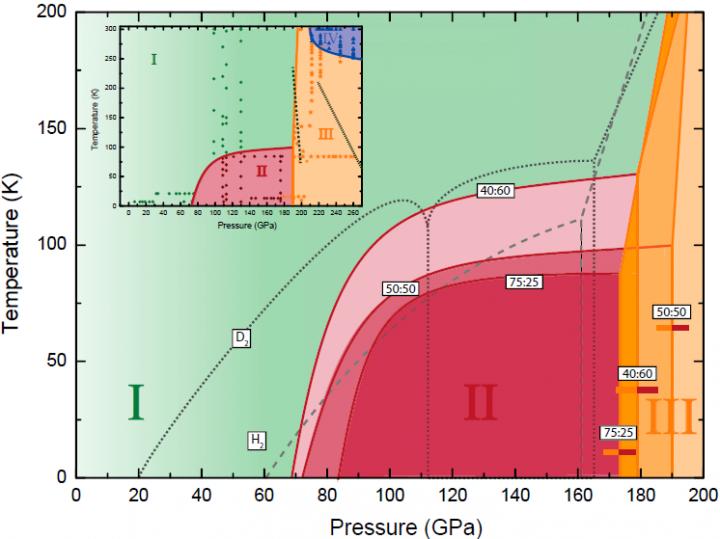
They found that mixtures of H2, HD, and D2 behaved as an isotopic molecular alloy (ideal solution) and exhibited symmetry-breaking phase transitions between phases I and II and phase III.
In their experiment, the researchers were surprised to find that all transitions occurred at higher pressures for the alloys than for either pure H2 or D2. This ran counter to any quantum effects based on isotope mass but could be explained by quantum trapping of high-kinetic energy states by the exchange interaction.
“Since HD has an intermediate mass and prevalent component in these alloys, one would expect that with its addition phase transitions would occur at intermediate P-T regimes”, said the leading scientist of this study, “The discrepancy from the more classical understanding of molecular phase diagrams, derives from the quantum nature of the hydrogen molecules themselves, where the exchange-symmetry can in effect trap the molecules in different, higher energy states.”
“HD molecules have no exchange symmetry, at low temperature all HD molecules will be in the lowest energy state. However, pure H2 and D2 have exchange symmetry, so some of the molecules would be trapped in the higher energy states. So the trapped kinetic energy is lower in mixtures than in either pure elements, and it shifts the phase transition to higher pressure in mixtures”, said LIU Xiaodi, the first author of the paper.
This work was supported by the National Natural Science Foundation of China, the CAS President’s International Fellowship Initiative, the Science Challenge Project, the CAS Innovation Fund and the Director’s Fund of Science Island.
###
Media Contact
ZHOU Shu
[email protected]
Original Source
http://english.
Related Journal Article
http://dx.




