Researchers from the University of Tsukuba demonstrate the direct conversion of scar tissue cells to heart muscle cells in mice after a heart attack
Tsukuba, Japan – It is estimated that during a heart attack, one billion cells in the heart are lost. In the wake of the heart attack, the lost tissue is replaced by scar tissue, which can lead to heart failure, arrhythmia and death. In a new study, researchers from the University of Tsukuba have shown how cells in the scar tissue can be converted to heart muscle cells, effectively regenerating the injured heart.
The injured heart of humans and rodents alike does not have the capacity to regenerate after injury. Therefore, the only way for the heart to heal the wound is to build a scar tissue in the injured area. A longstanding goal in the field has been to find a way to reprogram fibroblasts, cells that produce the connective tissue in a scar, to cardiomyocytes, the working heart muscle cells. By doing so, the lost heart muscle cells could be replaced, effectively preventing the heart from going into heart failure, a heart muscle weakness that can lead to death.
Previous studies have shown that cardiomyocytes appear to be formed by directly injecting a harmless virus carrying a set of cardiac transcription factors, proteins that drive the expression of genes that heart muscle cells need for their development and function, into the heart of rodents after a heart attack. However, the origin and functional significance of these newly formed heart muscle cells has not unequivocally been determined yet.
“Direct cardiac reprogramming holds great potential for cardiac regeneration and the treatment of myocardial infarction,” says lead author of the study Professor Masaki Ieda. “However, when transcription factors are introduced, apparent cardiomyocytes may be formed either by converting fibroblasts to new cardiomyocytes or by fusing fibroblasts with existing cardiomyocytes. The difference is that only the former process, which we call ‘direct reprogramming’, significantly contributes to regeneration. In this study, our goal was to determine how new cardiomyocytes are formed when cardiac transcription factors are introduced after myocardial infarction.”
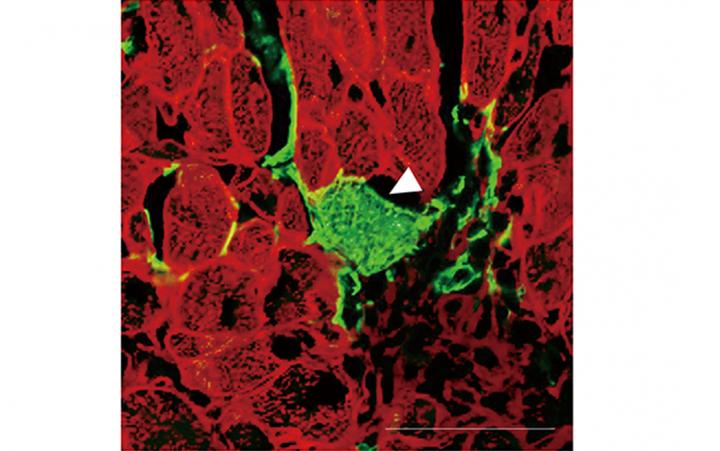
To achieve their goal, the researchers first generated mice in which all cells emitted red fluorescence. However, the mice were modified in a way that the fibroblasts emitted green fluorescence after treatment with the drug tamoxifen. As a result, when looking at the heart after treatment with tamoxifen, cells that emitted both red and green fluorescence indicated that cell fusion between fibroblasts and cardiomyocytes had happened. Conversely, the presence of green fluorescence indicated that direct reprogramming of fibroblasts to cardiomyocytes had occurred.
“These are striking results that show that fibroblasts can be directly reprogrammed to cardiomyocytes. Our findings demonstrate the exciting potential of direct reprograming as a strategy for cardiac regeneration after myocardial infarction,” says Professor Ieda.
###
The article, “Overexpression of Gata4, Mef2c, and Tbx5 Generates Induced Cardiomyocytes via Direct Reprogramming and Rare Fusion in the Heart” was published in Circulation at DOI: 10.1161/CIRCULATIONAHA.120.052799
Media Contact
Naoko Yamashina
[email protected]
Related Journal Article
http://dx.