In a remarkable breakthrough poised to redefine the frontiers of biological imaging, researchers have unveiled an advanced microscopy technique, termed confocal squared spinning-disk image scanning microscopy (C²SD-ISM). This innovation marries the speed and efficiency of spinning-disk confocal microscopy with the unparalleled resolution and contrast of image scanning microscopy, achieving high-fidelity super-resolution images of complex tissue structures like never before. The intricate interplay of optical engineering and computational synchronization heralds a new era for high-resolution visualization in biomedical sciences.
Central to this technique is the integration of a custom-designed spinning disk (SD) featuring pinholes artfully arranged in an Archimedean spiral pattern and controlled with exceptional precision. Mounted on a Nikon inverted fluorescence microscope, the system is adaptable across multiple magnifications, ranging from a 10× objective tailored for whole-animal imaging to a 100× high numerical aperture lens suited for ultra-detailed cellular studies. The high numerical aperture of 1.49 ensures enhanced light collection, critical for resolving minute structures within biological specimens.
What sets C²SD-ISM apart is its unique illumination and detection scheme. Illumination is provided by a multi-mode laser source capable of simultaneous multi-wavelength excitation essential for multicolor fluorescence imaging. The laser beam is homogenized and spatially modulated by a digital micromirror device (DMD), generating dynamic structured illumination patterns. This innovative use of the DMD not only shapes the excitation light with extreme fidelity but does so synchronously with image acquisition, ensuring that each frame corresponds precisely to a controlled illumination pattern.
.adsslot_zetrYNOCS5{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_zetrYNOCS5{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_zetrYNOCS5{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
The spinning disk, driven by a brushless motor at a remarkable 5000 revolutions per minute, serves as a rapid, rotating spatial filter. Its design enables the selective passage of in-focus light while rejecting out-of-focus background signals, effectively enhancing image contrast and depth discrimination. This high-speed rotation is finely coordinated with the camera exposure using a novel synchronization strategy, whereby the disk’s angular displacement during each exposure is tailored to an integer multiple of the pattern repetition angle. As a result, uniform and artifact-free imaging across the entire field of view is achieved without compromising temporal resolution.
Acquisition hardware coordination is orchestrated through sophisticated control software using analog and digital signals. The data acquisition card interfaces seamlessly with the DMD, sCMOS camera, piezoelectric sample stage, and excitation sources, allowing real-time modulation of illumination patterns, rapid image capture, and precise three-dimensional sample scanning. This tight integration underpins the system’s ability to perform volumetric super-resolution imaging rapidly, with minimal photobleaching and phototoxicity — a critical consideration for live-cell and tissue imaging.
To quantify the system’s performance, multiple evaluation metrics were employed, encompassing conventional measures like Michelson contrast along with more specialized indices such as local contrast (LC), fringe contrast (FC), and clarity ratio (CR). These metrics collectively assess the microscope’s prowess in rejecting out-of-focus fluorescence, enhancing feature visibility, and preserving image sharpness. Computed from grayscale intensity values and frequency-domain analysis, these parameters offer rigorous, multidimensional validation of imaging improvements attained by C²SD-ISM over conventional methods.
Beyond contrast and clarity, the fidelity of super-resolution reconstruction was meticulously quantified with peak signal-to-noise ratio (PSNR) and structural similarity index measure (SSIM). These metrics evaluate pixel-wise precision and perceptual congruity between reconstructed and reference images, respectively. Further analytical rigor was added through linear correlation analysis leveraging R squared (R²) statistics, demonstrating the high degree of correspondence between super-resolved images and their diffraction-limited counterparts after point spread function convolution.
To guard against reconstruction artifacts—a notorious challenge in computational microscopy—the team employed NanoJ-SQUIRREL, an advanced tool for assessing super-resolution image quality. This analysis provided residual error maps, resolution-scaled error (RSE), and resolution-scaled Pearson coefficient (RSP), highlighting the fidelity and reliability of the C²SD-ISM reconstructions. Complementing these analyses, Fourier ring correlation (FRC) and image decorrelation techniques quantitatively gauged the lateral resolution enhancements, affirming sub-diffraction imaging capabilities.
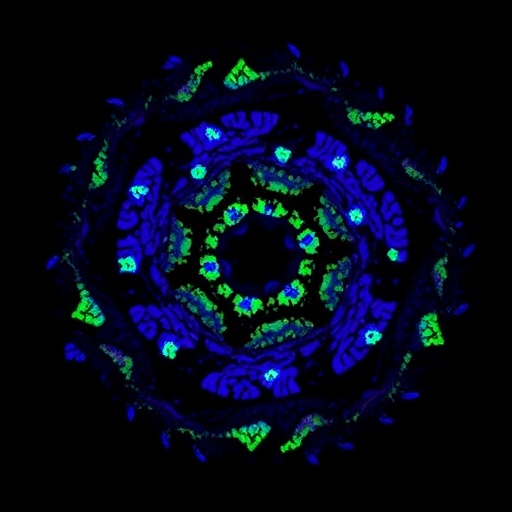
The power of this technique was vividly demonstrated in multicolor imaging of fixed biological samples, including mitochondria, F-actin, and nuclei within cultured cells, as well as complex tissue slices from mouse kidney and fungal specimens. With objectives optimized for different spatial scales, C²SD-ISM revealed exquisite structural detail with remarkable contrast and minimal background haze. The tri-color imaging experiments underscored its utility for multiplexed labeling studies, while the highly resolved kidney tissue images promise valuable insights for histopathology and organ-level analyses.
Notably, the system’s capacity for three-dimensional imaging was bolstered by a nano-positioning piezo sample scanner, enabling fine axial sectioning and volumetric reconstructions. Sample scanning, synchronized with patterned illumination and camera capture, permitted high-fidelity z-axis optical sectioning critical for dissecting complex tissue architectures. Such volumetric imaging holds great promise for studying dynamic biological processes and spatial relationships within intact specimens.
Post-acquisition processing capitalized on state-of-the-art deconvolution algorithms, specifically the Huygens software platform, which refined the raw data to remove residual blurring and optimize resolution. This computational refinement further enhanced the clarity and interpretability of super-resolution images, facilitating robust quantitative analysis and accurate visualization of subcellular structures.
The design ingenuity, synchronization precision, and image processing synergy embodied in C²SD-ISM collectively represent a leap forward in fluorescence microscopy. By marrying fast acquisition rates with confocal-level sectioning and super-resolution clarity, this method opens avenues for live biological imaging with unprecedented fidelity, speed, and multiplexing capabilities. The modular and programmable nature of the system also lends itself to customization and integration with emerging microscopy modalities.
Beyond academic research, the implications of C²SD-ISM extend to clinical diagnostics, drug discovery, and developmental biology, where detailed visualization of complex tissues at the nanoscale critically informs understanding of disease mechanisms and therapeutic responses. The demonstrated ability to handle multicolor samples across diverse biological contexts highlights its versatility and potential for broad adoption.
Importantly, the elegant solution to synchronizing spinning disk rotation, structured illumination patterning, and camera exposure exemplifies how intricate engineering challenges can be tackled to push optical microscopy boundaries. The team’s strategic use of analog and digital signals to coordinate heterogeneous hardware components underscores the growing importance of interdisciplinary approaches combining optics, electronics, and software.
While the current work focused on fixed samples for proof of principle and optimization, the underlying principles and demonstrated system capabilities pave the way for live-cell adaptations. The rapid image acquisition paired with reduced photodamage risk positions C²SD-ISM as a strong candidate for dynamic imaging of living specimens, tracking cellular processes with spatial and temporal precision.
As the microscopy landscape evolves, innovations like C²SD-ISM exemplify a broader trend emphasizing super-resolution, speed, and user-friendly operation. This elegant convergence empowers researchers to visualize biological phenomena at scales and speeds previously unattainable, deepening insights into cellular architecture and tissue physiology.
In sum, the confocal squared spinning-disk image scanning microscopy system epitomizes a transformative advance in fluorescence microscopy. Its harmonious blending of mechanical ingenuity, optical finesse, and computational refinement delivers unprecedented imaging performance, promising to catalyze discoveries across the life sciences. As it disseminates into laboratories worldwide, it is poised to be a cornerstone tool in the quest to unravel the complexities of biological systems with dazzling clarity.
Subject of Research: High-fidelity tissue super-resolution imaging using an advanced confocal squared spinning-disk image scanning microscopy technique.
Article Title: High-fidelity tissue super-resolution imaging achieved with confocal² spinning-disk image scanning microscopy.
Article References:
Liang, Q., Ren, W., Jin, B. et al. High-fidelity tissue super-resolution imaging achieved with confocal² spinning-disk image scanning microscopy. Light Sci Appl 14, 260 (2025). https://doi.org/10.1038/s41377-025-01930-x
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41377-025-01930-x
Tags: advanced biological imaging methodsbiomedical imaging innovationscomputational synchronization in imagingconfocal squared spinning-disk microscopyhigh numerical aperture lenseshigh-fidelity tissue imagingintricate tissue structure visualizationmulticolor fluorescence imagingNikon inverted fluorescence microscopeoptical engineering in microscopyspinning-disk confocal microscopysuper-resolution microscopy techniques