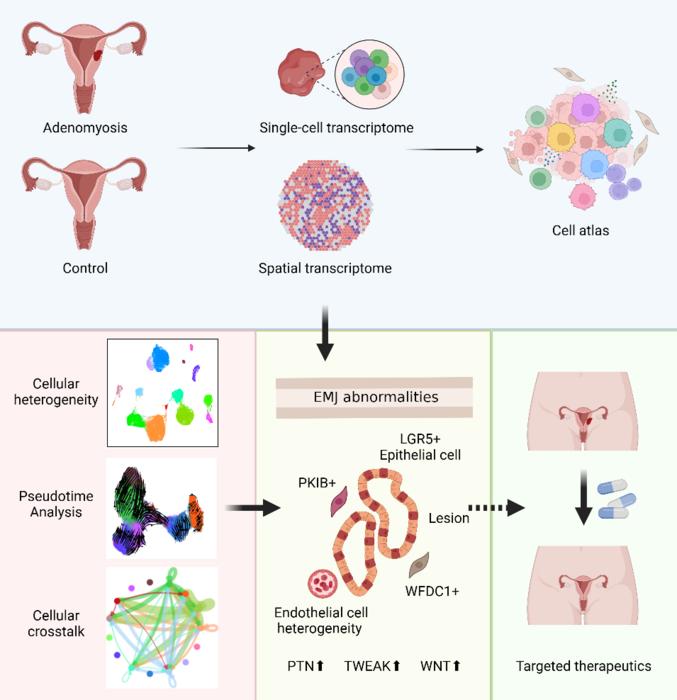
Adenomyosis is a poorly understood gynecological disorder with limited treatment options. The study employed single-cell RNA sequencing and spatial transcriptomics to map transcriptional alterations across different regions of the uterus in adenomyosis patients and controls. It highlights unique epithelial and stromal subpopulations and aberrant signaling pathways involved in adenomyosis, offering potential targets for precise diagnostics and therapeutics.
Credit: Tao Chen, Yiliang Xu, Xiaocui Xu, Jianzhang Wang, Zhiruo Qiu, Yayuan Yu, Xiaohong Jiang, Wanqi Shao, Dandan Bai, Mingzhu Wang, Shuyan Mei, Tao Cheng, Li Wu, Shaorong Gao, Xuan Che
Adenomyosis is a poorly understood gynecological disorder with limited treatment options. The study employed single-cell RNA sequencing and spatial transcriptomics to map transcriptional alterations across different regions of the uterus in adenomyosis patients and controls. It highlights unique epithelial and stromal subpopulations and aberrant signaling pathways involved in adenomyosis, offering potential targets for precise diagnostics and therapeutics.
Key findings from the study include:
- Unique epithelial (LGR5+) and stromal (PKIB+) subpopulations were identified in adenomyotic lesions, supporting a complex interplay between “invagination” and “metaplasia” theories. Additionally, WFDC1+ stromal progenitor cells might act as precursors to lesion-specific stromal cells, indicating a sophisticated mechanism of lesion formation.
- Abnormal angiogenic signaling and endothelial cell heterogeneity were observed in lesions. Notable pathways involved include vascular endothelial growth factor and angiopoietin, highlighting disrupted angiogenic processes in these tissues.
- Altered cell-cell communication was identified between ectopic and eutopic endometrium, particularly within adenomyotic lesions. The study found aberrant signaling pathways, including pleiotrophin, TWEAK, and WNT cascades, suggesting modified signaling dynamics in the lesions.
This study advances the understanding of adenomyosis by providing a comprehensive single-cell and spatial transcriptomic landscape. It reveals unique cellular subpopulations and signaling aberrations within adenomyotic lesions, supporting both invagination and metaplasia theories. These insights offer potential for developing precise diagnostic and therapeutic strategies, emphasizing the need for clinical validation of these findings. The work entitled “ Comprehensive transcriptional atlas of human adenomyosis deciphered by the integration of single-cell RNA-sequencing and spatial transcriptomics ” was published on Protein & Cell (published on Mar. 15, 2024).
Journal
Protein & Cell
DOI
10.1093/procel/pwae012
Method of Research
Experimental study
Subject of Research
Human tissue samples
Article Title
Comprehensive transcriptional atlas of human adenomyosis deciphered by the integration of single-cell RNA-sequencing and spatial transcriptomics
Article Publication Date
15-Mar-2024




