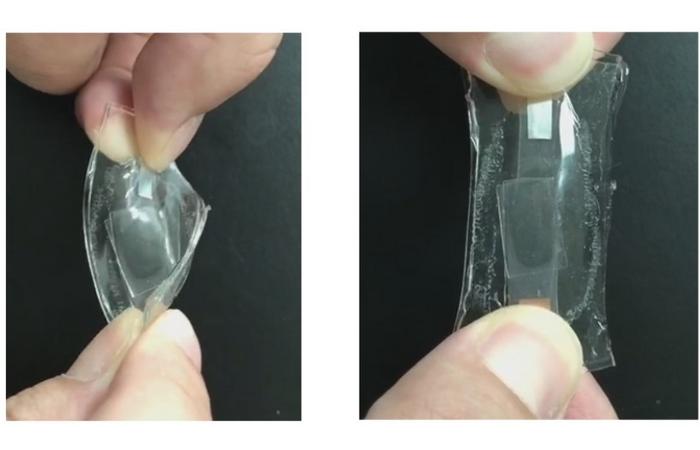
When you think of a battery, you probably don’t think stretchy. But batteries will need this shape-shifting quality to be incorporated into flexible electronics, which are gaining traction for wearable health monitors. Now, researchers in ACS Energy Letters report a lithium-ion battery with entirely stretchable components, including an electrolyte layer that can expand by 5000%, and it retains its charge storage capacity after nearly 70 charge/discharge cycles.
Credit: Adapted from ACS Energy Letters 2024, DOI: 10.1021/acsenergylett.4c01254
When you think of a battery, you probably don’t think stretchy. But batteries will need this shape-shifting quality to be incorporated into flexible electronics, which are gaining traction for wearable health monitors. Now, researchers in ACS Energy Letters report a lithium-ion battery with entirely stretchable components, including an electrolyte layer that can expand by 5000%, and it retains its charge storage capacity after nearly 70 charge/discharge cycles.
Electronics that bend and stretch need batteries with similar properties. Most researchers who have attempted to build such batteries created them with woven conductive fabric or rigid components folded into expandable shapes, similar to origami. But for a truly malleable battery, every part — the electrodes that collect charge and the charge-balancing middle electrolyte layer — must be elastic. So far, truly stretchy battery prototypes have moderate elasticity, complex assembly processes or limited energy storage capacity, especially over time with repeated charging and discharging. The latter can be due to a weak connection between the electrolyte layer and electrodes or instability of the fluid electrolyte, which can move around when the battery changes shape. So, rather than using a liquid, Wen-Yong Lai and coworkers wanted to incorporate the electrolyte into a polymer layer fused between two flexible electrode films, to create a completely solid, stretchy battery.
To make the electrodes for the fully elastic battery, the team spread a thin film of conductive paste containing silver nanowires, carbon black and lithium-based cathode or anode materials onto a plate. A layer of polydimethylsiloxane, a flexible material commonly used in contact lenses, was then applied to the top of the paste. Directly on top of this film, the researchers added a lithium salt, a highly conductive liquid and the ingredients to make a stretchy polymer. When activated by light, these components combined to form a solid, rubbery layer capable of stretching to 5000% of its original length and able to transport lithium ions. Finally, the stack was topped with another electrode film, and the whole device was sealed in a protective coating.
When comparing the solid stretchy battery design to a similar device with a traditional liquid electrolyte, the new version had about six times higher average charge capacity at a fast-charging rate. Likewise, the solid battery maintained a more stable capacity while operating during 67 charging and discharging cycles. In other prototypes made with solid electrodes, the polymer electrolyte maintained steady operation over 1000 cycles, with capacity dropping by 1% in the first 30 cycles, compared to a 16% drop for the liquid electrolyte. There are still improvements to be made, but this new way of creating fully stretchable, solid batteries could be a promising step forward for wearable or implantable devices that flex and move with the body.
The authors acknowledge funding from the National Key Research and Development Program of China; the National Natural Science Foundation of China; the Natural Science Foundation of Jiangsu Province; the Foundation of Key Laboratory Flexible Electronics of Zhejiang Province; Program for Jiangsu Specially-Appointed Professor; NUPT “1311 Project” and Scientific Foundation; China Postdoctoral Science Foundation; the Project of State Key Laboratory of Organic Electronics and Information Displays, NJUPT; and the Natural Science Foundation of NJUPT.
The paper’s abstract will be available on July 17 at 8 a.m. Eastern time here: http://pubs.acs.org/doi/abs/10.1021/acsenergylett.4c01254
For more of the latest research news, register for our upcoming meeting, ACS Fall 2024. Journalists and public information officers are encouraged to apply for complimentary press registration by completing this form.
###
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive news releases from the American Chemical Society, contact [email protected].
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Follow us: X, formerly Twitter | Facebook | LinkedIn | Instagram
Journal
ACS Energy Letters
DOI
10.1021/acsenergylett.4c01254
Article Title
Elastic Polymer Electrolytes Integrated with In Situ Polymerization-Transferred Electrodes toward Stretchable Batteries
Article Publication Date
17-Jul-2024




