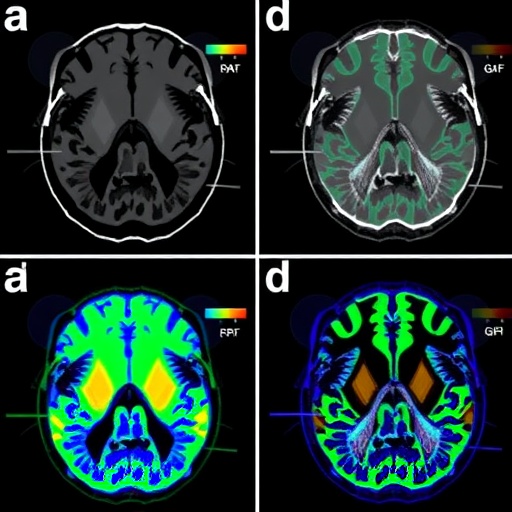
In a groundbreaking study shedding light on the intricacies of Alzheimer’s disease diagnosis and treatment, researchers have embarked on a comprehensive exploration of various radiopharmaceuticals labeled with fluorine-18 (18F). This innovative research, conducted by a dedicated team led by Park, BN., Kim, SM., and An, YS., showcases the potential of advanced imaging techniques using Positron Emission Tomography (PET) in the context of neurodegenerative disorders, particularly Alzheimer’s disease. The study meticulously evaluates the comparative efficacy of different 18F-labeled PET agents in visualizing amyloid plaques and tau protein deposits, hallmark features of Alzheimer’s pathology, in a murine model that closely mimics the human condition.
As the global population ages, Alzheimer’s disease has emerged as one of the foremost challenges facing healthcare systems worldwide. Characterized by memory loss, cognitive decline, and behavioral issues, the progressive nature of Alzheimer’s necessitates early detection and effective monitoring strategies. Traditional diagnostic techniques often fall short, primarily due to the disease’s insidious onset. Consequently, researchers have turned to advanced imaging modalities like PET, which utilize radiolabeled compounds to visualize biological processes in living organisms. This study focuses primarily on the 18F-labeled radiotracers, which have shown significant promise in this arena.
The primary aim of the research was to conduct a comparative analysis of various 18F-labeled PET radiopharmaceuticals and their effectiveness in imaging key pathological features of Alzheimer’s disease in a prey model. These radiopharmaceuticals potentially provide a non-invasive method to assess disease progression and might guide therapeutic interventions. The mouse model employed in this study exhibits pathophysiological features akin to human Alzheimer’s disease, including progressive amyloidosis and tau pathology. By employing such models, researchers can attain deeper insights into the molecular underpinnings of the disease.
One of the most compelling aspects of this study is the meticulous attention paid to the selection of radiopharmaceuticals. The researchers examined a suite of candidates, evaluating their pharmacokinetic properties, binding affinity to amyloid and tau deposits, and overall imaging efficacy. Each radiopharmaceutical was evaluated for its ability to traverse the blood-brain barrier, an essential characteristic for any compound intended for neuronal imaging. The study systematically highlighted how each radiotracer performed against established benchmarks, paving the way for future diagnostic practices.
Understanding the mechanisms by which these 18F-labeled agents bind to Alzheimer’s-related pathology is crucial. Alpha-amino acids, peptides, and small-molecule inhibitors are common structures being investigated. Each compound undergoes rigorous evaluation concerning its binding affinity, specificity, and overall imaging signal in the context of PET scans. Through quantitative analysis, the study illustrates the nuances that differentiate each radiopharmaceutical, providing future researchers and clinicians a clear framework to guide their endeavors.
In addition to pharmacokinetics, the research extends its scope by examining dosimetry and safety profiles of the various compounds. Given the increasing prevalence of PET imaging in neurodegenerative disease management, it is vital to understand the radiation exposure risks associated with these tracers. The findings reveal crucial data that will influence clinical decision-making and patient safety standards. By meticulously documenting dosimetric data, the research compiles evidence that could lead to the refinement of guidelines in clinical settings where these imaging agents are employed.
The study’s findings indicate pronounced differences in the imaging capabilities of the various 18F-labeled radiopharmaceuticals. Some tracers demonstrated superior binding to amyloid plaques, while others showcased enhanced visualization of tau tangles, underscoring the disease’s complexity. This multifaceted approach to imaging not only enhances our understanding of disease mechanisms but opens avenues for personalized medicine applications. Clinicians might leverage these differences to tailor imaging strategies to reflect the specific pathophysiological features predominant in individual patients.
Moreover, the implications of this study stretch beyond mere visualization. By discerning which 18F-labeled agents yield the most accurate representations of Alzheimer’s pathology, the research provides a path to optimizing treatment protocols. Future therapeutic strategies could couple efficacy with imaging, thereby augmenting understanding of treatment impacts on underlying disease processes.
Research on Alzheimer’s disease has traditionally faced hurdles regarding translation into clinical applications. However, the emphasis on clarity and specificity in this study serves to bridge the knowledge gap. The identification of promising 18F-labeled PET radiopharmaceuticals could expedite their path to clinical trials and, eventually, real-world implementation. As pharmaceutical companies seek to develop effective therapies, understanding the imaging landscape becomes paramount, and this research contributes significantly to that endeavor.
The broader scientific community stands to benefit from the insights garnered in this compelling comparative analysis. With the rich data sets emerging from advanced imaging studies, researchers worldwide can utilize the findings to further explore the role of neuroimaging in myriad neurodegenerative conditions. As the fight against Alzheimer’s continues, fostering collaboration across institutions will be crucial in refining these imaging techniques and enhancing patient care.
As the study sets the stage for future advances, it lays a robust framework for investigating additional radiopharmaceuticals and imaging methodologies. The potential inclusion of novel compounds, alongside ongoing refinements of existing agents, posits that this field will continue to evolve rapidly. The quest for optimal imaging strategies in Alzheimer’s disease has taken a significant leap forward, with the research conducted by Park, BN., Kim, SM., and An, YS., serving as a cornerstone reference for years to come.
In conclusion, the comparative study on 18F-labeled PET radiopharmaceuticals not only enhances our diagnostic capabilities in Alzheimer’s disease but also acts as a vital resource for future research into effective therapeutic strategies. These findings signify hope for millions affected by this debilitating disease, potentially paving the way for timely interventions and improved patient outcomes. The landscapes of neuroimaging and neuropharmacology are set to transform, thanks to innovation and dedication in this crucial area of biomedical research.
Subject of Research: Alzheimer’s disease and radiopharmaceutical imaging techniques.
Article Title: Comparative study of 18F-labeled PET radiopharmaceuticals in an Alzheimer’s disease mouse model.
Article References:
Park, BN., Kim, SM. & An, YS. Comparative study of 18F-labeled PET radiopharmaceuticals in an Alzheimer’s disease mouse model. BMC Neurosci 26, 55 (2025). https://doi.org/10.1186/s12868-025-00978-0
Image Credits: AI Generated
DOI:
Keywords: Alzheimer’s disease, PET imaging, radiopharmaceuticals, fluorine-18, neurodegeneration, amyloid, tau, mouse model, diagnostic techniques, pharmacokinetics.
Tags: 18F PET imaging in Alzheimer’s researchadvanced imaging modalities for diagnosisAlzheimer’s disease healthcare challengesamyloid plaques visualization in micecognitive decline diagnostic methodscomparative analysis of radiopharmaceuticalsearly detection of Alzheimer’s diseaseefficacy of 18F-labeled PET agentsmurine models in Alzheimer’s studiesneurodegenerative disorders imaging techniquespositron emission tomography applicationstau protein deposits detection methods