Credit: Alisa Weigandt for Duke Health
DURHAM, N.C. – Breast cancer cells that carry a certain gene mutation can be induced to die using a combination of an existing targeted therapy along with an investigational molecule tested by Duke Cancer Institute researchers.
When used together in preclinical experiments, the drugs shut down two of the key survival strategies these types of cancer cells use to evade treatment.
The finding in mouse models and human tumor cells has wide implications for advancing treatment if planned clinical trials prove the approach successful. About 35 percent of breast cancers have this gene mutation when they are discovered, and even more develop the mutation after exposure to standard treatments, which could make them susceptible to the combination approach.
"This work reflects several careful studies to not only define the mechanism of action of the combination therapy, but also to explore the therapy's activity in cellular and animal models," said Kris C. Wood, Ph.D., assistant professor in the Department of Pharmacology and Cancer Biology at Duke and senior author of the study published Dec. 14 in the journal Science Translational Medicine.
"Our preliminarily findings suggest that this therapy may be a safe and effective approach in human breast cancer patients who carry this mutation," Wood said.
Wood and colleagues, including lead author Grace R. Anderson, began their investigation with a medical contradiction: Breast tumors proliferate in part because the regulatory processes that control cell growth and death go awry. Several drugs inhibit these regulatory pathways, but they have shown modest results in solid tumors, including breast cancers.
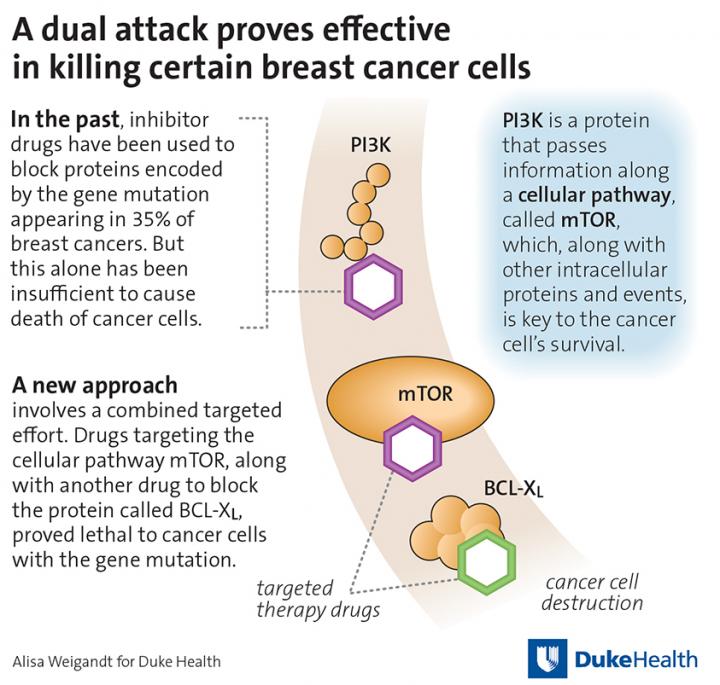
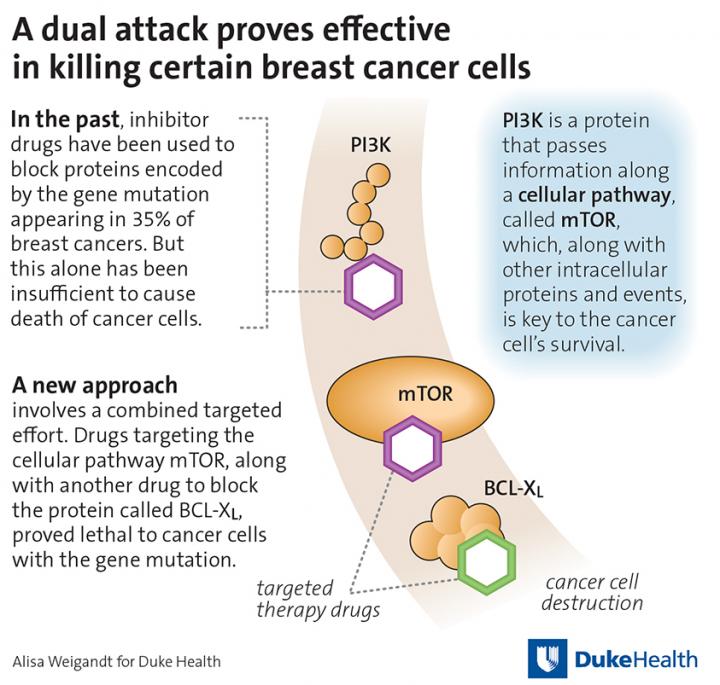
In their study, the Duke researchers began by asking how to make one investigational drug work more effectively. The drug, called ABT-737, inhibits a cellular pathway involved in programmed cell death. Consistent with other studies, the agent had little effect on a variety of solid cancer tumor cells the researchers tested.
But when the researchers added a second agent — this time a drug that inhibits another cell survival pathway called the mammalian target of rapamycin, or mTOR, pathway — a subset of breast cancer cells died at a substantial rate.
This set of breast cancer cells all carry a mutation in the PIK3CA gene, which is found in about 35 percent of newly diagnosed breast cancer tumors. The PIK3CA gene encodes a protein called PI3K, which directly activates the mTOR pathway. Previous studies have shown that breast cancers with PIK3CA gene mutations fail to respond well to drugs either targeting PI3K or mTOR for reasons that are largely unknown.
Through further study, the researchers appear to have solved this conundrum. They found that PIK3CA-mutated tumors are uniquely able to compensate when either of the test therapies — ABT-737 or an mTOR inhibitor — is used alone, relying on the cell survival pathway that isn't targeted by the drug to stay alive. But when used in combination, the drugs become lethal, shutting down both cell survival pathways and enabling the tumor cells to die.
"One of the compelling results of the study is that we can use very low doses of both therapies to achieve strong results," said lead author Anderson. "At such low doses, the side effects could be minimized."
The researchers said studies are being planned to advance the findings in clinical trials using currently approved therapies or investigational drugs like Navitoclax, a clinical analog of ABT-737.
###
In addition to Wood and Anderson, study authors include Suzanne E. Wardell, Merve Cakir, Lorin Crawford, Jim C. Leeds, Daniel P. Nussbaum, Pallavi S. Shankar, Ryan S. Soderquist, Elizabeth M. Stein, Jennifer P. Tingley, Peter S. Winter, Elizabeth K. Zieser-Misenheimer, Holly M. Alley, Alexander Yllanes, Victoria Haney, Kimberly L. Blackwell, Shannon J. McCall, and Donald P. McDonnell.
The study received federal funding from the National Institutes of Health (K12HD043446, DK48807), the Department of Defense (BC151664), and the National Science Foundation (DGE-1106401, DGF-1106401). Full grant support is listed in the publication.
Media Contact
Sarah Avery
[email protected]
919-660-1306
@DukeHealth
http://www.dukehealthnews.org
############
Story Source: Materials provided by Scienmag