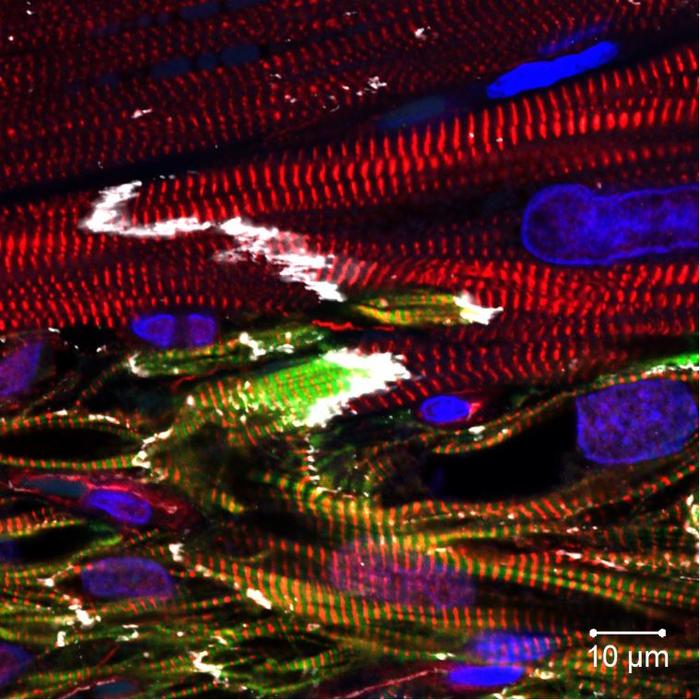
Researchers at the University of Wisconsin–Madison and Academia Sinica of Taiwan have harnessed a combination of lab-grown cells to regenerate damaged heart muscle.
Credit: Yu-Che Cheng
Researchers at the University of Wisconsin–Madison and Academia Sinica of Taiwan have harnessed a combination of lab-grown cells to regenerate damaged heart muscle.
The study, published in Circulation — which addresses major challenges of using heart muscle cells, called cardiomyocytes, grown from stem cells — takes a crucial step toward future clinical applications.
Previous research has shown that transplanting cardiomyocytes made from induced pluripotent stem cells (iPSC) can replace muscle in the hearts of mammals. Researchers have struggled to bring the treatment to the clinic, in part because the implanted cells haven’t developed enough life-sustaining blood vessels to survive very long.
The new study confronted that challenge by combining the lab-grown cardiomyocytes with stem-cell-derived endothelial cells — the cells that line blood. The combination therapy also holds promise for tackling arrhythmia, another significant obstacle in heart regeneration with stem-cell-derived cardiomyocytes.
“Our findings suggest that human iPSC-derived endothelial cells can effectively augment the remuscularization of the heart by iPSC-derived cardiomyocytes, offering a promising avenue for future clinical applications,” says Patrick Hsieh, a researcher with Academia Sinica’s Institute of Biomedical Sciences who conducted the study while working as a visiting professor at the UW–Madison Stem Cell & Regenerative Medicine Center.
Hsieh and study lead author Yu-Che Cheng collaborated with Tim Kamp, who serves as director of the Stem Cell & Regenerative Medicine Center, as well as a team of researchers at UW–Madison and the Wisconsin National Primate Research Center, to examine the therapeutic effect of co-transplantation in mice and non-human primates undergoing a heart attack.
“The main advantage of iPSCs is their ability to be differentiated into many types of cells and serve as a valuable resource for cell therapy,” says Cheng, who is a project manager at Academia Sinica. “In this study, we generated billions of endothelial cells and cardiomyocytes from the same iPSCs line to inject into mice and non-human primates.”
“The simple idea of the project was to enhance blood flow and promote survival of iPSC-cardiomyocytes using blood vessel-forming endothelial cells,” Kamp says. “But the reality of generating the optimal cell preparations followed by precise delivery to the heart reflects tremendous effort by an international team of collaborators.”
The team would like to conduct further studies to refine their cell transplantation protocols and assess long-term safety and efficacy. Promising results, Hsieh believes, would lead to clinical trials with human patients with heart disease, a leading cause of death around the world.
“As a cardiac surgeon now focusing on translational research, the most exciting aspect of this research is the potential to make a meaningful impact on the treatment of heart disease,” Hsieh says. “Witnessing the significant improvements in cardiac function and tissue regeneration resulting from our combined cell therapy approach is both inspiring and promising for the future of cardiovascular medicine.”
This research was supported by grants from the Ministry of Science and Technology, Taiwan (111-2321-B-001-012, 111-2740-B-001-003, 110-2320-B-001-023), National Health Research Institutes (EX111-10907SI), U.S. National Academy of Medicine and Academia Sinica (AS-HLGC-109-05), National Institutes of Health (U01HL134764 and UL1TR002373) and the National Science Foundation (EEC-1648035).
Researchers at the University of Wisconsin–Madison and Academia Sinica of Taiwan have harnessed a combination of lab-grown cells to regenerate damaged heart muscle.
The study, published in Circulation — which addresses major challenges of using heart muscle cells, called cardiomyocytes, grown from stem cells — takes a crucial step toward future clinical applications.
Previous research has shown that transplanting cardiomyocytes made from induced pluripotent stem cells (iPSC) can replace muscle in the hearts of mammals. Researchers have struggled to bring the treatment to the clinic, in part because the implanted cells haven’t developed enough life-sustaining blood vessels to survive very long.
The new study confronted that challenge by combining the lab-grown cardiomyocytes with stem-cell-derived endothelial cells — the cells that line blood. The combination therapy also holds promise for tackling arrhythmia, another significant obstacle in heart regeneration with stem-cell-derived cardiomyocytes.
“Our findings suggest that human iPSC-derived endothelial cells can effectively augment the remuscularization of the heart by iPSC-derived cardiomyocytes, offering a promising avenue for future clinical applications,” says Patrick Hsieh, a researcher with Academia Sinica’s Institute of Biomedical Sciences who conducted the study while working as a visiting professor at the UW–Madison Stem Cell & Regenerative Medicine Center.
Hsieh and study lead author Yu-Che Cheng collaborated with Tim Kamp, who serves as director of the Stem Cell & Regenerative Medicine Center, as well as a team of researchers at UW–Madison and the Wisconsin National Primate Research Center, to examine the therapeutic effect of co-transplantation in mice and non-human primates undergoing a heart attack.
“The main advantage of iPSCs is their ability to be differentiated into many types of cells and serve as a valuable resource for cell therapy,” says Cheng, who is a project manager at Academia Sinica. “In this study, we generated billions of endothelial cells and cardiomyocytes from the same iPSCs line to inject into mice and non-human primates.”
“The simple idea of the project was to enhance blood flow and promote survival of iPSC-cardiomyocytes using blood vessel-forming endothelial cells,” Kamp says. “But the reality of generating the optimal cell preparations followed by precise delivery to the heart reflects tremendous effort by an international team of collaborators.”
The team would like to conduct further studies to refine their cell transplantation protocols and assess long-term safety and efficacy. Promising results, Hsieh believes, would lead to clinical trials with human patients with heart disease, a leading cause of death around the world.
“As a cardiac surgeon now focusing on translational research, the most exciting aspect of this research is the potential to make a meaningful impact on the treatment of heart disease,” Hsieh says. “Witnessing the significant improvements in cardiac function and tissue regeneration resulting from our combined cell therapy approach is both inspiring and promising for the future of cardiovascular medicine.”
This research was supported by grants from the Ministry of Science and Technology, Taiwan (111-2321-B-001-012, 111-2740-B-001-003, 110-2320-B-001-023), National Health Research Institutes (EX111-10907SI), U.S. National Academy of Medicine and Academia Sinica (AS-HLGC-109-05), National Institutes of Health (U01HL134764 and UL1TR002373) and the National Science Foundation (EEC-1648035).
Journal
Circulation
DOI
10.1161/CIRCULATIONAHA.122.061736
Article Title
Combined Treatment of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes and Endothelial Cells Regenerate the Infarcted Heart in Mice and Non-Human Primates
Article Publication Date
21-Sep-2023




