In recent years, the ubiquity of synthetic chemicals in our environment has prompted growing concerns regarding their impacts on human health and development. One such pervasive compound, Bisphenol A (BPA), widely utilized in manufacturing plastics, has been under intense scrutiny due to its potential as an endocrine disruptor. Endocrine-disrupting chemicals (EDCs) like BPA are capable of interfering with hormone systems, leading to adverse developmental, reproductive, neurological, and immune effects in both wildlife and humans. Yet, despite the widespread acknowledgment of BPA’s endocrine-disrupting properties, the precise molecular mechanisms driving its multifaceted impact remain only partially elucidated. A groundbreaking study led by Professor Tatsuyuki Takada at Ritsumeikan University presents compelling experimental evidence linking BPA’s neurodevelopmental toxicity to its interaction with retinoic acid (RA) signaling pathways, with profound implications for understanding chemical exposure risks during early gestation.
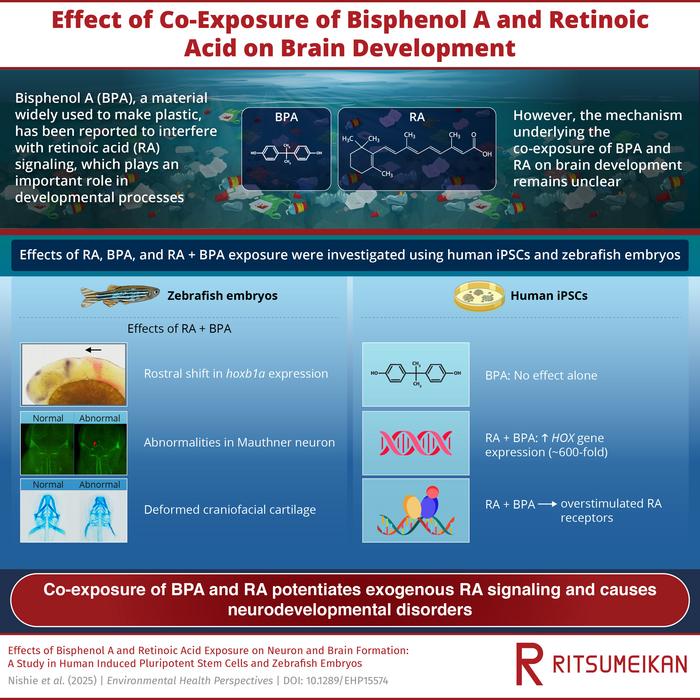
To probe how the simultaneous presence of BPA and RA impacts neurodevelopment, Takada’s team employed a dual experimental approach leveraging human induced pluripotent stem cells (iPSCs) and zebrafish embryos, an established vertebrate model whose transparent embryogenesis facilitates direct observation of morphological and molecular changes. Their meticulous exposure paradigms compared the consequences of BPA alone, RA alone, and combined BPA-RA treatments on gene expression and developmental endpoints. Strikingly, BPA in isolation did not significantly perturb developmental processes; however, when co-administered with RA, BPA markedly amplified RA signaling. This synergistic overactivation was especially evident in the dysregulation of HOX gene clusters, master regulators of anterior-posterior body axis patterning.
.adsslot_o5PmwJl0rY{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_o5PmwJl0rY{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_o5PmwJl0rY{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
HOX genes, evolutionarily conserved transcription factors, dictate spatial identity and organ positioning during embryogenesis. The observed upregulation of HOX genes under BPA-RA co-exposure precipitated aberrant brain and craniofacial formation, as evidenced by morphological abnormalities in zebrafish neuroanatomy and facial structures. Such malformations included the rostral displacement of neural domain markers like hoxb1a and duplication of specific neuronal populations, notably Mauthner cells, which are critical for motor reflexes. These developmental perturbations mimic phenotypes reminiscent of human neurodevelopmental disorders such as autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD), underscoring a potential environmental etiology linked to chemical co-exposures.
Fundamental to understanding the mechanistic underpinning of this synergy, the researchers demonstrated through pharmacological interventions that blocking RA receptors significantly mitigated the BPA-induced potentiation of RA signaling. This provides critical evidence that BPA acts through modulation of the RA signaling cascade rather than through its previously characterized estrogenic pathways. The implications of this finding are profound, challenging the singular focus on classical steroid hormone receptors in BPA toxicity and expanding the horizon to nutrient-associated signal transduction pathways as targets of endocrine disruption.
The intersection of environmental chemicals and nutrient-derived signaling molecules represents a novel frontier in toxicology and developmental biology. Retinoic acid’s centrality in early embryonic patterning makes its aberrant activation a sensitive readout for developmental insults. BPA’s ability to amplify RA pathway activity suggests that combined chemical-nutrient exposures, even at low environmental levels previously deemed safe individually, may synergize to disrupt fundamental developmental processes. This challenges current regulatory frameworks that often assess chemical hazards in isolation, highlighting the necessity of integrated evaluations considering co-exposures and complex mixture effects.
Water systems worldwide have documented the presence of BPA due to leaching from consumer product packaging, thermal receipts, and household items; concomitantly, low concentrations of RA-like compounds have been identified in drinking water sources. This environmental co-occurrence signifies realistic exposure scenarios for human populations, especially pregnant women and developing fetuses. The vulnerability of early developmental stages to chemical perturbations is well recognized, and these new findings spotlight a hitherto unrecognized axis of risk involving BPA and RA interactions.
Moreover, the study’s use of human iPSCs offers a translational bridge from animal models to human biology, providing an in vitro platform to dissect molecular responses within a human cellular context. These pluripotent cells mirror early developmental stages and enable the monitoring of neuronal differentiation trajectories under chemical influences. Observing that BPA potentiated RA-induced gene expression alterations and morphological changes in iPSC-derived neural progenitors further strengthens the relevance of these findings to human developmental health.
Professor Takada emphasizes that this research “illuminates the complex crosstalk between environmental chemicals and endogenous signaling networks, revealing a critical pathway through which BPA can exert neurodevelopmental toxicity.” The study thereby elevates the importance of considering how ubiquitous environmental chemicals may modulate physiologically essential pathways with far-reaching consequences across organ systems.
This work urges policymakers, regulators, and public health experts to reexamine permissible exposure limits and risk assessment procedures, taking into account combinatorial interactions between chemicals and nutrients. Greater surveillance of drinking water quality for RA-like activity and stricter controls on BPA usage could be essential steps to protect vulnerable populations from developmental neurotoxicity. Furthermore, it signals a call to researchers to deepen investigations into endocrine disruption beyond classical receptor paradigms, appraising the broader molecular circuitry susceptible to environmental insults.
As synthetic chemical production escalates globally, unearthing mechanistic insights such as those provided by Takada and colleagues becomes indispensable to safeguarding developmental integrity and mitigating the burden of neurodevelopmental disorders linked to environmental factors. Their pioneering approach sheds light on the pleiotropic effects of endocrine disruptors across multiple signaling axes, reinforcing the complexity of chemical exposures in real-world scenarios.
Ultimately, this study positions retinoic acid signaling as a central node through which the combined exposure to BPA and RA operates to modify neuronal and brain development. The implications resonate beyond basic science, compelling a redefinition of how chemical safety is evaluated and prompting holistic strategies to minimize harmful exposures during critical windows of human development.
Subject of Research: Animals
Article Title: Effects of Bisphenol A and Retinoic Acid Exposure on Neuron and Brain Formation: A Study in Human Induced Pluripotent Stem Cells and Zebrafish Embryos
News Publication Date: 13-May-2025
Web References:
– Environmental Health Perspectives article: https://doi.org/10.1289/EHP15574
– Ritsumeikan University: http://en.ritsumei.ac.jp/
– Ritsumeikan University Research Report: https://www.ritsumei.ac.jp/research/radiant/eng/
References:
DOI: 10.1289/EHP15574
Image Credits: Prof. Tatsuyuki Takada from Ritsumeikan University, Japan
Keywords: Brain development, Developmental neuroscience, Developmental biology, Biochemistry, Toxicology, Stem cells, Molecular biology, Retinoic acid, Gene expression, Developmental disorders, Environmental health, Public health
Tags: Bisphenol A impact on brain developmentchemical exposure during early gestationeffects of environmental chemicals on developmentendocrine disruptors and human healthendocrine-disrupting chemicals researchhormone receptor interactions with BPAimplications of BPA in reproductive healthmolecular mechanisms of BPA toxicityneurodevelopmental toxicity of BPAretinoic acid signaling pathwaysRitsumeikan University BPA studysynthetic chemicals and neurological effects