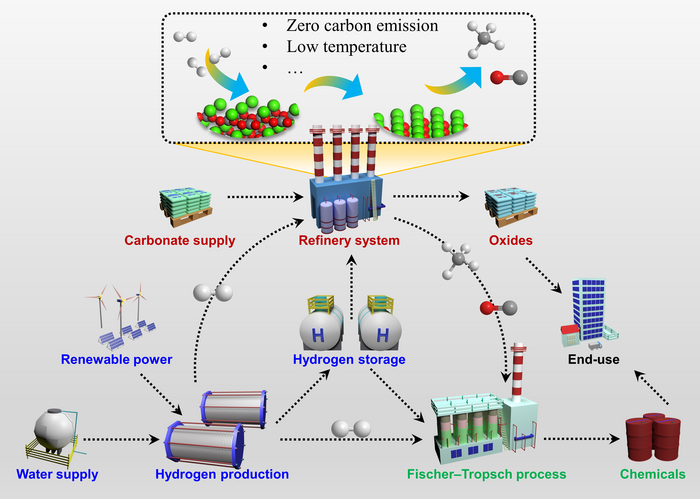
This study is led by Prof. Mingfei Shao and Prof. Xue Duan (College of Chemistry, Beijing University of Chemical Technology). Interestingly, the beginning decomposition temperature of CaCO3 in hydrogen atmosphere is clearly reduced (as low as 600 °C) and the emission of CO2 was largely inhibited. Instead, gaseous CO was produced with a high selectivity of 95.8% and the reaction rate could be up to 0.756 mmol min−1. Meanwhile, the high-purity and porous solid CaO was produced, which has the great application potentials. This study demonstrated that the CO results from the selective cleavage of Ca–O bonds at the surface of CaCO3 via the direct hydrogenation mechanism at relatively low temperature. However, it undergoes the reverse water-gas shift (RWGS) reaction path at high temperature, i.e., CO being produced by the reduction of CO2 released by the decomposition of carbonates.
Credit: ©Science China Press
This study is led by Prof. Mingfei Shao and Prof. Xue Duan (College of Chemistry, Beijing University of Chemical Technology). Interestingly, the beginning decomposition temperature of CaCO3 in hydrogen atmosphere is clearly reduced (as low as 600 °C) and the emission of CO2 was largely inhibited. Instead, gaseous CO was produced with a high selectivity of 95.8% and the reaction rate could be up to 0.756 mmol min−1. Meanwhile, the high-purity and porous solid CaO was produced, which has the great application potentials. This study demonstrated that the CO results from the selective cleavage of Ca–O bonds at the surface of CaCO3 via the direct hydrogenation mechanism at relatively low temperature. However, it undergoes the reverse water-gas shift (RWGS) reaction path at high temperature, i.e., CO being produced by the reduction of CO2 released by the decomposition of carbonates.
“The hydrogenation of metal carbonates gives a potential chance to change the carbon transfer pathway in order to inhibit CO2 emission and obtain high value-added gaseous products (such as CO). Co-thermal via residual heats generated by carbonate decomposition, coupling the thermal decomposition of carbonate and reduction process will make a huge breakthrough. we are surprised that in-situ hydrogenation of carbonates coupled with a green hydrogen system not only makes full use of energy, but also solves the storage, transport, and safety problems of hydrogen.” Shao says.
A few implications thus emerge for designing the system for Co-thermal in-situ reduction of inorganic carbonates: 1) Hydrogenation mechanism and carbon transfer path are of great significance for emission reduction and efficiency enhancement; 2) Through the design and development of the co-thermal in-situ reduction process toward high value-added products will produce huge social and environmental benefits.
See the article:
Co-thermal in-situ reduction of inorganic carbonates to reduce carbon-dioxide emission
https://doi.org/10.1007/s11426-022-1537-6
Journal
Science China Chemistry
DOI
10.1007/s11426-022-1537-6




