Credit: NINS/IMS
The research group including researchers of Exploratory Research Center on Life and Living Systems (ExCELLS), Institute for Molecular Science (IMS) in National Institutes of Natural Sciences and Osaka University have revealed the detail mechanism of the biosynthesis of carbon monoxide essential for the maturation of the active site of NiFe-hydrogenase.
Background:
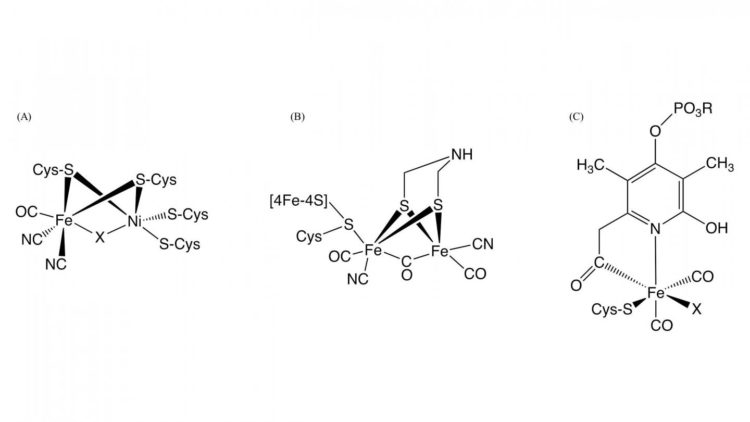
Hydrogenase, which is a metalloenzyme responsible for the oxidation of hydrogen gas and the reduction of proton, plays a key role of bacterial hydrogen metabolism. Based on the differences of metal content of the active site, they are classified into three groups; NiFe-, FeFe-, and Fe-hydrogenases containing different metal complexes as active centers in these enzymes (Figure 1). Although the structures of the active centers of them are different, it is essential for hydrogenase activity that carbon monoxide (CO) is coordinated to the iron ion in the active center. It is known that CO is biosynthesized by an enzymatic reaction, but the detail of CO biosynthesis was unknown.
Result:
In this research, the research group determined the crystal structure of the enzyme (HypX) responsible for the biosynthesis of CO (Figure 2), based on which HypX biosynthesizes CO by unprecedented reaction for the maturation of NiFe-hydrogenase. HypX consists of two domains; the N-terminal and C-terminal domains. A continuous cavity connecting the N- and C-terminal domains is present in the interior of HypX (Figure 2). In the crystal structure, coenzyme A (CoA) is bound in the C-terminal region of the cavity.
Two different reactions occur in the N- and C-terminal domains. In the N-terminal domain, formyl-group transfer reaction from formyltetrahydrofolate, which is bound in the N-terminal region of the cavity as a substrate to CoA, takes place (reaction step 1 in Fig. 3). At this time, CoA in the cavity adopts the linear extended conformation, and the SH-group of CoA is located near the formyl group in the formyltetrahydrofolate bound in the N-terminal domain. Then, formyl-CoA is produced as a reaction intermediate by the formyl-group transfer reaction from formyltetrahydrofolate to CoA.
In the next step, formyl-CoA undergoes a large conformational change in the cavity so that the formyl group in the terminal position of formyl-CoA is located at the active site in the C-terminal domain of HypX (reaction step 2 in Fig. 3). In the C-terminal domain, CO is formed by decarbonylation of formyl-CoA (reaction step 3 in Fig. 3).
This CO biosynthesis reaction is the unprecedented and novel reaction. CoA is well known as a coenzyme, which has the important role in fatty acid metabolism and cellular energetic metabolism through the citric acid cycle. However, it has never been reported that CoA/formyl-CoA is involved in CO biosynthesis reactions. This research has revealed a novel physiological function of a well-known coenzyme CoA.
Future prospects:
The biosynthetic mechanisms of metalloenzymes remain unknown in many cases. It remains to be elucidated especially how the metal-containing active centers of metalloenzymes have been assembled. In this work, we determined the first crystal structure of the enzyme that catalyzes the biosynthetic reaction of carbon monoxide essential for the construction of the active site of [NiFe] hydrogenase. In future, we will continue the research for elucidating the detail mechanism of whole hydrogenase maturation pathway based on this result.
###
Media Contact
Shigetoshi Aono
[email protected]
81-564-595-575
Original Source
https:/
Related Journal Article
http://dx.