Credit: IBS
Although most of our electronic devices, like mobile phones, laptops and electric vehicles use lithium rechargeable batteries, what is going on inside them is not fully understood. Researchers from the Center for Molecular Spectroscopy and Dynamics, within the Institute for Basic Science (IBS) succeeded in observing in realtime the ultrafast dynamics of lithium ions with femtosecond time resolution (1/1,000,000,000,000,000 of a second). These findings help to explain what happens during the process of charging and discharging: a cornerstone for the advanced batteries with better performance. Published on Nature Communications, this study reveals the interactions between lithium ions and electrolytes, which are organic molecules that surround the lithium ions and conduct electricity, and were able to conclude that the existing theory on ion diffusion in lithium rechargeable batteries is not completely correct.
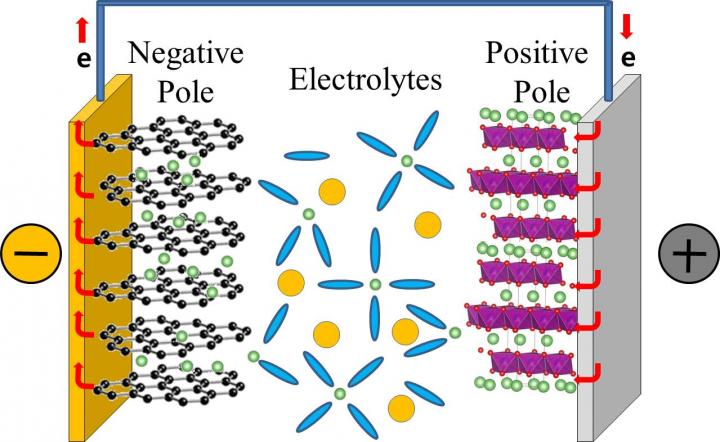
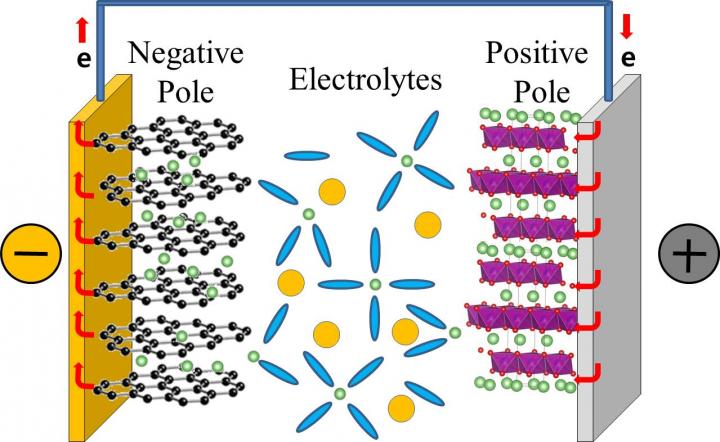
In a typical commercial lithium rechargeable battery, lithium ions dissolved in electrolytes move from the positive to the negative pole of the battery when the battery is charging. And, they migrate in the opposite way, when the battery is in use. The lithium ion mobility determines the performance of the lithium rechargeable battery, and determines how rapidly they can charge and discharge.
Lithium ions, however, do not migrate alone, they are surrounded by electrolytes that facilitate the journey from one pole to the other. Currently, the electrolytes in our lithium rechargeable batteries are typically composed of a mixture of: ethylene carbonate (EC), dimethyl carbonate (DMC), and diethyl carbonate (DEC) in equal concentration. It is believed that lithium ions associate mainly with EC, forming the so-called "solvation shell" or "solvation sheath", while DMC and DEC are just enhancing the movement of these shells between the batteries' poles, like "lubricants". However, while most of the previous studies focused on the static properties of the bond between electrolytes and lithium ions, this study clarifies the dynamics of the bonding. Like in a motion picture, where a series of still images displayed rapidly one after the other create the effect of movements, IBS scientists took quick shots to analyze the formation and breaking of these bonds. However, while movies are typically filmed and displayed at 24 still images per seconds, these measurement "shots" were taken at time intervals of just femtoseconds.
Thanks to a tool called two-dimensional infrared spectroscopy, the team measured how lithium ions bind to the oxygen atoms of the DEC and found that these bonds break and form in a matter of 2-17 picoseconds (1/1,000,000,000,000 of a second). The timescale is similar for DMC. This means that DMC and DEC are more than just "lubricants", they are also part of the solvation shell together with EC and may play an active role in transporting lithium ions to the battery's pole.
"It was believed that EC makes a rigid shell around lithium ions during the migration between electrodes. However, this study shows that the solvent shell is not that rigid, it is constantly restructured during the ion transport," explains Professor CHO Minhaeng. And concludes: "For this reason, revising the existing lithium ion diffusion theory is inevitable."
The research team is working on a follow-up study to establish a new theory of the lithium ion diffusion process and it is building a new ultra-high-speed laser spectroscopy instrument that can observe the chemical reaction as well as film it on top of the rechargeable batteries' electrodes.
###
Media Contact
Dahee Carol Kim
[email protected]
@IBS_media
http://www.ibs.re.kr/en/
############
Story Source: Materials provided by Scienmag