A new discovery by researchers at the RIKEN Center for Biosystems Dynamics (BDR) in Japan upends decades of assumptions regarding DNA replication. Led by Ichiro Hiratani and colleagues, the experiments published August 28 in Nature show that DNA replication in early embryos is different from what past research has taught, and includes a period of instability that is prone to chromosomal copying errors. As failed pregnancies and developmental disorders are often related to chromosomal abnormalities the findings could impact the field of reproductive medicine, perhaps leading to improved methods of in vitro fertilization (IVF).
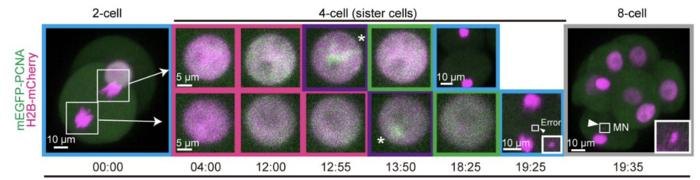
During embryogenesis, the initially fertilized egg divides, as do each new set of daughter cells. By the third day after fertilization, an embryo has undergone three divisions and contains 16 cells. Each cell division is accompanied by DNA replication, ensuring that each daughter cell contains a copy of the whole genome. In their new study, the team of RIKEN BDR researchers set out to characterize the nature of the DNA replication process in early-stage embryos. They used their homemade single-cell genomics technique called scRepli-seq and applied it to developing mouse embryos. With this technology, the team was able to take snapshots of single embryonic cell DNA at different times during the DNA-replication periods. What they found contradicted what scientists have assumed about DNA replication in embryos.
“We found multiple specialized types of DNA replication during early mouse embryogenesis, which no one has seen before,” says Hiratani. “In addition, we also found that at certain points, genomic DNA is temporarily unstable and chromosomal aberrations are elevated.”
Textbooks tell us that DNA doesn’t replicate all at once. Instead, different regions of a chromosome are duplicated in a specific sequence. The team’s first discovery was that the replication-timing domains observed in mature cells do not exist until an embryo has 4 cells. This means that unlike any other cells in an eventual body, DNA is replicated uniformly, not sequentially, in 1- and 2-cell embryos.
Each time a part of a chromosome unwinds for replication, regions of DNA unzip forming a structure that looks like a fork in the road. For replication to proceed, the fork must move down the strand of DNA, rezipping copied regions and unzipping the next section. The team’s second discovery was that fork speed is much slower in the 1, 2, and 4-cell stages than after the 8-cell stage of embryogenesis. The 4-cell embryo can now be seen as a transitional stage during which uniform DNA replication becomes sequential, while still showing slow fork movement characteristic of 1- and 2-cell embryos. In contrast, 8-cell embryos are much more similar to mature cells, showing sequential replication and fast fork movement.
Errors in DNA replication in the first few days after fertilization often result in chromosome irregularities, such as extra copies, missing copies, breaks in copies, or incomplete copies. Some of these copying mistakes lead to miscarriage, while others lead to developmental disorders like Down Syndrome, also known as trisomy 21. The team’s third discovery was that the frequency of chromosomal copying errors was temporarily elevated in early-stage embryos, most commonly during the 4-cell stage.
The researchers again used scRepli-seq, this time for detecting chromosome copy number abnormalities. They found that very few errors occurred during the transition between 1- and 2-cell stages or between 8- and 16-cell stages. On the other hand, 13% of cells showed chromosomal abnormalities during the transition between the 4- and 8-cell stages, likely due to copying errors during the 4-cell stage. Further testing suggested that the copying errors at this stage were related to the slow-moving forks.
“Our findings lead to many new questions,” says Hiratani. “For example, are these series of phenomena evolutionarily conserved in other species, including human embryos? And what are the subsequent fates of cells with chromosomal aberrations?” In addition to guiding basic research in the future, this discovery could help fertilization clinics devise better strategies for minimizing the chromosomal abnormalities that are common in the first few days after fertilization.
A new discovery by researchers at the RIKEN Center for Biosystems Dynamics (BDR) in Japan upends decades of assumptions regarding DNA replication. Led by Ichiro Hiratani and colleagues, the experiments published August 28 in Nature show that DNA replication in early embryos is different from what past research has taught, and includes a period of instability that is prone to chromosomal copying errors. As failed pregnancies and developmental disorders are often related to chromosomal abnormalities the findings could impact the field of reproductive medicine, perhaps leading to improved methods of in vitro fertilization (IVF).
During embryogenesis, the initially fertilized egg divides, as do each new set of daughter cells. By the third day after fertilization, an embryo has undergone three divisions and contains 16 cells. Each cell division is accompanied by DNA replication, ensuring that each daughter cell contains a copy of the whole genome. In their new study, the team of RIKEN BDR researchers set out to characterize the nature of the DNA replication process in early-stage embryos. They used their homemade single-cell genomics technique called scRepli-seq and applied it to developing mouse embryos. With this technology, the team was able to take snapshots of single embryonic cell DNA at different times during the DNA-replication periods. What they found contradicted what scientists have assumed about DNA replication in embryos.
“We found multiple specialized types of DNA replication during early mouse embryogenesis, which no one has seen before,” says Hiratani. “In addition, we also found that at certain points, genomic DNA is temporarily unstable and chromosomal aberrations are elevated.”
Textbooks tell us that DNA doesn’t replicate all at once. Instead, different regions of a chromosome are duplicated in a specific sequence. The team’s first discovery was that the replication-timing domains observed in mature cells do not exist until an embryo has 4 cells. This means that unlike any other cells in an eventual body, DNA is replicated uniformly, not sequentially, in 1- and 2-cell embryos.
Each time a part of a chromosome unwinds for replication, regions of DNA unzip forming a structure that looks like a fork in the road. For replication to proceed, the fork must move down the strand of DNA, rezipping copied regions and unzipping the next section. The team’s second discovery was that fork speed is much slower in the 1, 2, and 4-cell stages than after the 8-cell stage of embryogenesis. The 4-cell embryo can now be seen as a transitional stage during which uniform DNA replication becomes sequential, while still showing slow fork movement characteristic of 1- and 2-cell embryos. In contrast, 8-cell embryos are much more similar to mature cells, showing sequential replication and fast fork movement.
Errors in DNA replication in the first few days after fertilization often result in chromosome irregularities, such as extra copies, missing copies, breaks in copies, or incomplete copies. Some of these copying mistakes lead to miscarriage, while others lead to developmental disorders like Down Syndrome, also known as trisomy 21. The team’s third discovery was that the frequency of chromosomal copying errors was temporarily elevated in early-stage embryos, most commonly during the 4-cell stage.
The researchers again used scRepli-seq, this time for detecting chromosome copy number abnormalities. They found that very few errors occurred during the transition between 1- and 2-cell stages or between 8- and 16-cell stages. On the other hand, 13% of cells showed chromosomal abnormalities during the transition between the 4- and 8-cell stages, likely due to copying errors during the 4-cell stage. Further testing suggested that the copying errors at this stage were related to the slow-moving forks.
“Our findings lead to many new questions,” says Hiratani. “For example, are these series of phenomena evolutionarily conserved in other species, including human embryos? And what are the subsequent fates of cells with chromosomal aberrations?” In addition to guiding basic research in the future, this discovery could help fertilization clinics devise better strategies for minimizing the chromosomal abnormalities that are common in the first few days after fertilization.
Journal
Nature
DOI
10.1038/s41586-024-07841-y