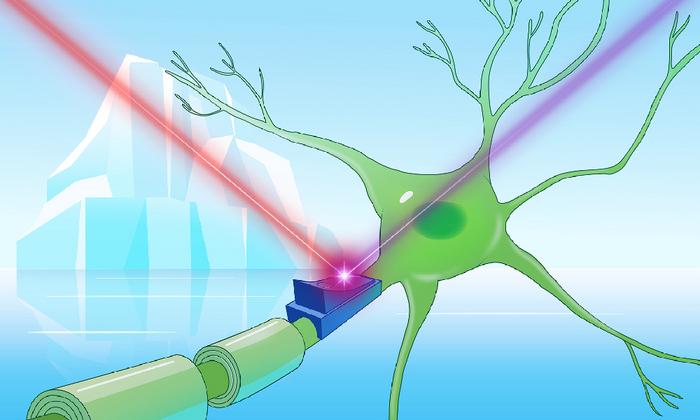
In the vast, frozen realms of Earth’s coldest environments—ranging from the sprawling glaciers of Greenland and the pristine icy aquifers of Finland to the lofty Tibetan plateaus—lurks a remarkable group of proteins poised to revolutionize neuroscience and cellular biology. These proteins, dubbed cryorhodopsins, are newly identified microbial rhodopsins that defy previous understanding by thriving exclusively in frigid habitats and exhibiting unique structural and functional features. At the heart of this discovery is Kirill Kovalev, an innovative postdoctoral researcher affiliated with EMBL Hamburg and EMBL-EBI, whose interdisciplinary expertise bridges physics and structural biology to unravel the complexities of light-sensitive proteins in extremophiles.
Rhodopsins have long fascinated scientists due to their role as light-activated proteins, primarily known for enabling aquatic microorganisms to harness energy from sunlight and for their pivotal applications in optogenetics—the technique of manipulating neuronal activity with light. However, Kovalev’s investigation into protein databases revealed an intriguing anomaly: a subset of rhodopsins isolated strictly from extraordinarily cold ecosystems bore striking similarities despite vast geographical separation. This surprising genetic and structural conservation hinted at a specialized adaptation strategy, prompting the christening of this family as “cryorhodopsins,” signaling their chillingly unique ecological niche.
.adsslot_YejmnZluaE{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_YejmnZluaE{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_YejmnZluaE{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Kovalev and his collaborators delved deeper, applying cutting-edge structural biology techniques including X-ray crystallography and cryo-electron microscopy under controlled light activation to map the atomic architecture of these proteins at unprecedented resolution. These analyses revealed a subtle but critical rearrangement in the retinal-binding pocket of cryorhodopsins responsible for their blue-shifted absorption properties. By deciphering the atomic-level modifications that confer such optical properties, the team has opened the door to rational design of synthetic blue rhodopsins tailor-made for advanced biomedical and research tools.
Functional assays in cultured neurons further illuminated the dual-switch capabilities of cryorhodopsins. Upon UV light exposure, cells expressing these proteins exhibited inward electrical currents, indicative of activation, whereas sequential illumination with green or red light modulated cellular excitability in opposite directions. This bidirectional control introduces an unprecedented level of finesse to optogenetic manipulation, potentially enabling refined toggling of neural circuits with applications spanning fundamental neuroscience, therapeutic development, and bioengineering.
Beyond their photochemical roles, cryorhodopsins appear to double as sophisticated UV light sensors. Spectroscopic investigations spearheaded by Goethe University Frankfurt scientists uncovered the extremely slow photodynamic response kinetics of cryorhodopsins relative to canonical variants. Such temporal dynamics are characteristic of sensory rather than purely phototransductive proteins, suggesting that these rhodopsins might function as molecular sentinels warning microbes of deleterious UV exposure common in high-altitude or snow-embedded environments.
A particularly groundbreaking aspect of this research is the discovery of a physically coupled small protein whose gene co-localizes with cryorhodopsin genes. Employing artificial intelligence-driven protein structure prediction tools such as AlphaFold, the team proposed a pentameric ring assembly of this minor protein interfacing intimately with the rhodopsin. The working hypothesis posits that upon UV light reception by cryorhodopsin, the small protein acts as a messenger, relocating within the cell to propagate the signal internally. This intricate mechanism exemplifies a sophisticated molecular communication system evolved in microorganisms to survive and adapt in extreme habitats.
The evolutionary impetus behind the puzzling presence and dual functionality of cryorhodopsins remains an open question. Kovalev speculates that rather than cold per se driving these adaptations, it is the intense UV radiation that often accompanies frosty, high-elevation environments that served as the selective force. Thus, these proteins may represent a defensive evolutionary innovation, enabling microbes to detect and respond to harmful radiation exposures, thereby enhancing survival in otherwise hostile ecological niches.
Unlocking these insights was not without formidable obstacles. Cryorhodopsins’ near-identical sequence and structural homogeneity mean that even picometer-scale atomic shifts can dramatically alter their properties, necessitating the use of 4D structural biology techniques integrating time-resolved crystallography and cryo-EM to capture dynamic photoactivation states. Such precision experimental frameworks were vital for revealing how minute structural nuances translate into functional diversity.
Moreover, the proteins’ extreme photosensitivity demanded meticulous sample handling and data acquisition under near-total darkness to prevent premature activation. Collaboration across multiple international research institutions, coupled with access to specialized beamlines like EMBL Hamburg’s P14, were essential components enabling the successful structural characterization and functional assays of these elusive molecules.
While cryorhodopsins have yet to be harnessed as practical optogenetic tools, their early characterization as “cellular power switches” sets a compelling precedent. Kovalev envisions future engineered variants optimized for high efficiency, reversible control, and compatibility with deep tissue applications. Such advancements hold the promise to transform neuroscience research and pave the way for innovative therapeutic approaches, including improved optical cochlear implants and interventions in neurological disorders.
The discovery of cryorhodopsins epitomizes the transformative potential of combining bioinformatics, AI-driven modeling, advanced structural techniques, and functional validation in living cells. It also underscores the importance of exploring remote and extreme environments, where nature’s molecular ingenuity often reveals novel biotechnological treasures waiting to be uncovered and harnessed for human benefit.
Subject of Research: Cells
Article Title: CryoRhodopsins: a comprehensive characterization of a group of microbial rhodopsins from cold environments
News Publication Date: 4-Jul-2025
Web References: http://dx.doi.org/10.1126/sciadv.adv1015
Image Credits: Daniela Velasco/EMBL
Keywords: Microbiology, Signal transduction, Cell biology
Tags: cold-adapted proteinscryorhodopsinsecological adaptations of proteinsextremophiles in biologyfrozen environments of Earthinterdisciplinary studies in biologyKirill Kovalev researchlight-sensitive proteinsmicrobial rhodopsinsneuroscience applicationsoptogenetics in researchstructural biology of proteins