Chemists from Freiburg have succeeded in converting polynuclear transition metal carbonyls into their homoleptic complex cations using typical inorganic oxidants. In their work, the research team of Malte Sellin, Christian Friedmann and Prof. Dr. Ingo Krossing from the Institute of Inorganic and Analytical Chemistry and Maximilian Mayländer and Sabine Richert from the Institute of Physical Chemistry at the University of Freiburg show that the anthracene derivative with a half-step potential of 1.42 Volts vs. Fc0/+ can be converted to the radical deelectronating salt by a nitrosonium salt. “We have thus pushed the frontier of fundamental research in coordination chemistry as well as in organometallic chemistry a bit further,” Krossing said. The research group published their findings in the journal Chemical Science.
Credit: provided by the research group
Chemists from Freiburg have succeeded in converting polynuclear transition metal carbonyls into their homoleptic complex cations using typical inorganic oxidants. In their work, the research team of Malte Sellin, Christian Friedmann and Prof. Dr. Ingo Krossing from the Institute of Inorganic and Analytical Chemistry and Maximilian Mayländer and Sabine Richert from the Institute of Physical Chemistry at the University of Freiburg show that the anthracene derivative with a half-step potential of 1.42 Volts vs. Fc0/+ can be converted to the radical deelectronating salt by a nitrosonium salt. “We have thus pushed the frontier of fundamental research in coordination chemistry as well as in organometallic chemistry a bit further,” Krossing said. The research group published their findings in the journal Chemical Science.
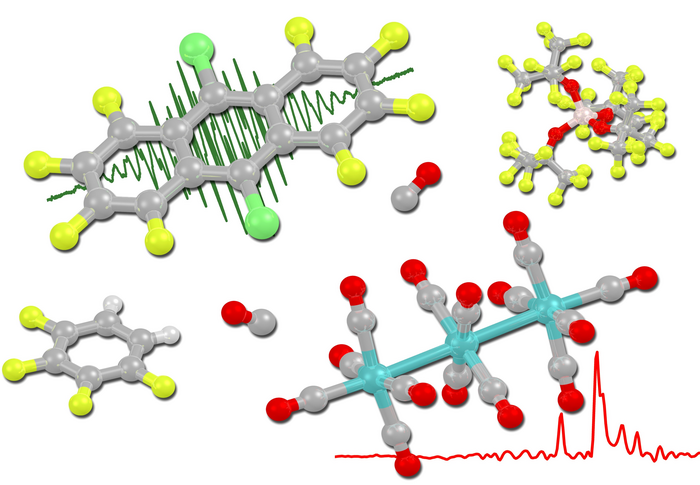
Deelectronator made from commercial chemical
In order to gain access to the hitherto almost unknown class of clustered transition metal carbonyl cations, chemists at the University of Freiburg have been looking for a way to ionize substrates without triggering undesired side reactions. During ionization, a neutral molecule loses one or more electrons. As a result, a positively charged molecule, also called a cation, is formed. A so-called innocent deelectronator is an ionizing agent that only accepts electrons from the substrate and otherwise shows no other undesirable reactivities. Since the only innocent deelectronator known to date, a perfluorinated ammoniumyl cation, requires laborious and time-consuming synthesis, the Freiburg scientists have developed an alternative that is produced directly from a commercially available chemical: The anthracene derivative, with a half-step potential of 1.42 volts vs. Fc0/+, can be converted to the radical deelectronator salt by a nitrosonium salt. “The deelectronating salt allows us to remove electrons from the system while preserving the structure. So it’s particularly mild and creates systems that we haven’t been able to represent before. In the long term, these could help us to produce better catalysts,” Krossing explains.
The perhalogenated anthracene deelectronator is putative
First, the research group tried to generate the desired transition metal carbonyl cations by reacting trimetal dodecacarbonyls with a silver salt as oxidant. Direct reaction of the trimetal dodecacarbonyls with nitrosyl cations also failed to produce the hoped-for result. “However, if the nitrosyl cation is reacted in advance with a perhalogenated anthracene derivative, then the resulting acene radical cation deelectronates the trimetal dodecacarbonyls under carbon monoxide atmosphere and leads to the desired salts,” Sellin explains. “Until now, no one has succeeded in converting polynuclear transition metal carbonyls into their homoleptic complex cations by typical inorganic oxidants. We have now shown that it is possible,” says Krossing. Sellin adds, “Surprisingly, structural characterization as well as vibrational and nuclear magnetic resonance spectroscopies of our new cluster point to three strongly electronically different carbonyl ligands. It surprised us to see such different electronic behavior of virtually the same ligands in one molecule.”
Fact Overview:
- Prof. Dr. Ingo Krossing heads the Chair of Molecular and Coordination Chemistry at the Institute of Inorganic and Analytical Chemistry at the University of Freiburg and is a member of the Living, Adaptive and Energy-autonomous Materials Systems (livMatS) Cluster of Excellence.
- Krossing has received an Advanced Grant from the European Research Council (ERC) for his project “InnoChem – Innocent Deelectronation Chemistry,” in which he is conducting research on a universally valid redox scale.
- In 2018, Krossing was admitted to the Heidelberg Academy of Sciences and Humanities, and in 2020 to the Leopoldina National Academy of Sciences.
- Krossing’s research focuses on: weakly coordinating anions, reactive cations, catalysis for energy conversion, unified acidity and redox scales, and battery electrolytes and materials. More on Ingo Krossing’s research.
- Original publication: Sellin, M., Friedmann, C., Mayländer, M., Richert, S., Krossing, I. (2022): Towards clustered carbonyl cations [M3(CO)14]2+ (M = Ru, Os): the need for innocent deelectronation. Chemical Science. DOI: 10.1039/d2sc02358j
Contact:
Prof. Dr. Ingo Krossing
Institute for Inorganic and Analytical Chemistry
University of Freiburg
Phone: +49 (0)761/203-6122
E-Mail: [email protected]
Franziska Becker
Office of University and Science Communications
University of Freiburg
Phone: +49 (0)761/203-54271
E-Mail: [email protected]
Journal
Chemical Science
DOI
10.1039/d2sc02358j