Credit: Jin-Quan Yu/Scripps Research
LA JOLLA, CA – June 18, 2018 – Chemists at Scripps Research have addressed one of the most formidable challenges in synthetic chemistry by inventing a method for "enantioselective remote meta-CH activation," which enables the making of chiral molecules that were previously difficult or impossible to synthesize.
The method, reported today in Nature, is likely to be adopted widely for the making of prospective drugs and other chemical products.
"This new method should allow us to explore a large 'chemical space' that had been essentially off-limits," says Jin-Quan Yu, PhD, senior investigator and Frank and Bertha Hupp Professor of Chemistry at Scripps Research.
Chiral molecules are asymmetric, with "right hand" and "left hand" forms. Often only one of these forms (called enantiomers) has the desired biological or chemical activity, while the other is inert or even has unwanted side effects–and most ordinary reactions yield an impure, 50:50 mix of both.
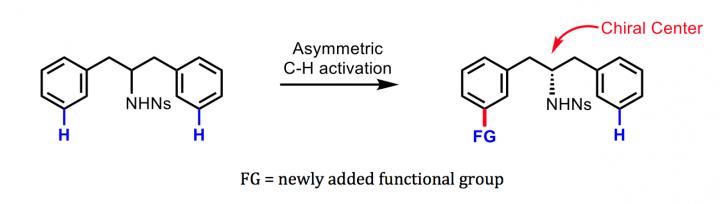
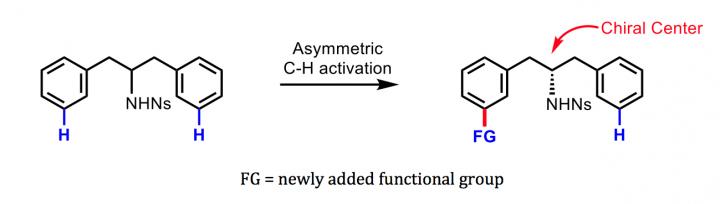
There are methods for turning a symmetric molecule into a chiral one and obtaining pure quantities of one enantiomer rather than the other. However, these methods typically involve the attachment of a reactive cluster of atoms called a functional group to the starting molecule at the point that becomes the center of asymmetry: the so-called chiral center. The new method attaches a new functional group relatively far from the chiral center–a feat previously achievable only by enzymes in living cells. Since the chiral center typically contains another functional group, the resulting chiral molecule ends up with two widely spaced functional groups, potentially conferring unique and potent bioactivity.
"The chiral molecules we can make with this method can be designed to interact with widely spaced binding sites on a target protein, for example," Yu says.
Key to the new method is a specially designed helper molecule, a "transient chiral mediator," based on the organic compound norbornene. It enables the crucial step of attaching the new functional group asymmetrically to an initially symmetric starting compound–far from the chiral center on the molecular backbone but, even so, yielding nearly 100 percent pure quantities of the desired enantiomer.
Yu's team demonstrated the technique by using it for the "remote chiral induction" of benzylamines and phenylethyl amines, broad classes of molecules that form the bases for many modern drugs as well as many biologically active compounds in plant and animal cells. The resulting chiral molecules typically comprised more than 95 percent of the desired enantiomer and less than 5 percent of the unwanted enantiomer.
Yu and his group currently are exploring ways to widen the scope of this strategy to other classes of starting molecule. They are also using their new method to create large libraries of previously inaccessible compounds, which can be screened to discover potential new drugs.
###
The first author of the paper, "Enantioselective Remote Meta-C?H Arylation and Alkylation via a Chiral Transient Mediator," was Hang Shi, a postdoctoral research associate in the Yu laboratory. The other co-authors were Alastair N. Herron, Ying Shao and Qian Shao of Scripps Research.
The study was funded by the National Institutes of Health (grant R01GM102265).
About Scripps Research
Scripps Research is one of the world's preeminent independent, not-for-profit organizations focusing on research in the biomedical sciences. Scripps Research is internationally recognized for its contributions to science and health, including its role in laying the foundation for new treatments for cancer, rheumatoid arthritis, hemophilia, and other diseases. An institution that evolved from the Scripps Metabolic Clinic founded by philanthropist Ellen Browning Scripps in 1924, the institute now employs more than 2,500 people on its campuses in La Jolla, CA, and Jupiter, FL, where its renowned scientists–including two Nobel laureates and 20 members of the National Academies of Science, Engineering or Medicine–work toward their next discoveries. The institute's graduate program, which awards PhD degrees in biology and chemistry, ranks among the top ten of its kind in the nation. For more information, see http://www.scripps.edu.
Media Contact
Madeline McCurry-Schmidt
[email protected]
858-784-9254
@scrippsresearch
http://www.scripps.edu