Phosphorus and its compounds are essential components of life and indispensable in our daily lives. In the human body, this element plays a crucial role in energy transfer and numerous cellular functions. Phosphorus compounds are used in fertilizers, detergents, medications, and many other products. Additionally, phosphorus is an essential ingredient in flame retardants, battery electrolytes, and catalysts. On Earth, phosphorus exists exclusively in the form of phosphates. The production of phosphorus-containing chemicals typically involves a complex and energy-intensive multi-step process. Initially, highly toxic white phosphorus (P4) is produced via a redox pathway and then further processed into phosphorus trichloride (PCl3) and other problematic and sometimes highly toxic intermediate products. Phosphorus chemistry based on P4 is associated with significant challenges but plays an indispensable role in the chemical industry due to its great importance.
Credit: Weigand Group
Phosphorus and its compounds are essential components of life and indispensable in our daily lives. In the human body, this element plays a crucial role in energy transfer and numerous cellular functions. Phosphorus compounds are used in fertilizers, detergents, medications, and many other products. Additionally, phosphorus is an essential ingredient in flame retardants, battery electrolytes, and catalysts. On Earth, phosphorus exists exclusively in the form of phosphates. The production of phosphorus-containing chemicals typically involves a complex and energy-intensive multi-step process. Initially, highly toxic white phosphorus (P4) is produced via a redox pathway and then further processed into phosphorus trichloride (PCl3) and other problematic and sometimes highly toxic intermediate products. Phosphorus chemistry based on P4 is associated with significant challenges but plays an indispensable role in the chemical industry due to its great importance.
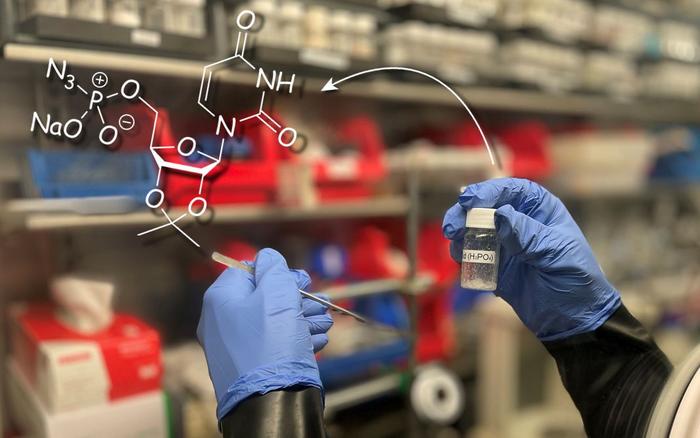
In the recent publication in the prestigious journal Nature Synthesis, Jan J. Weigand, Professor of Inorganic Molecular Chemistry at TU Dresden, along with his team, presents a remarkably simple method for the production of phosphorus-containing chemicals. This process directly converts primary and secondary phosphate sources into phosphorus-containing chemicals in just two steps, without using hazardous intermediates such as white phosphorus (P4). Jan J. Weigand explains, “The publication of the new process is a real highlight of my scientific career and the result of more than 15 years of research work. We have developed a blueprint for a much more sustainable phosphorus chemistry. Our process is extremely relevant for industrial applications due to its simplicity in terms of resources, costs, and time. We have already filed two patents, one of which has already been disclosed. Our new synthesis pathway enables greater independence from third countries since Europe no longer possesses a production facility for P4, which remains an indispensable intermediate in industrial phosphorus chemistry. Currently, Europe relies on production in countries like Vietnam and China. Our process can ensure resilience in the production of phosphorus-containing chemicals in the future,” explains Jan J. Weigand, highlighting the innovative nature of the process.
First author Tobias Schneider describes the procedure of the new method: “We have successfully produced a versatile phosphorylation reagent ((Pyridine)2PO2[OTf]) using a simple chemical reaction in which two oxygen atoms in phosphates are replaced by a labile ligand such as pyridine. This reagent allows for a redox-neutral approach to a variety of important phosphorus-containing chemicals with various applications by readily reacting with various nucleophiles such as amines, alcohols, or pseudohalides. The process enables a more direct and energy-efficient value chain by utilizing cost-effective raw materials such as phosphoric acid or other phosphate sources, thus bypassing the use of white phosphorus as an intermediate.”
Prof. Weigand’s team is currently working on expanding the range of phosphorus-containing chemicals producible with the novel method and recycling the chemicals necessary for the process through electrochemical methods to develop an efficient circular process. This approach allows for further conservation of resources and cost savings.
Original publication:
Schneider, Tobias., Schwedtmann, Kai., Fidelius, Jannis, & Weigand, Jan J. Redox-neutral conversion of ubiquitous PV sources to a versatile PO2+ phosphorylation reagent. Nature Synthesis (2023). https://doi.org/10.1038/s44160-023-00344-0
Journal
Nature Synthesis
DOI
10.1038/s44160-023-00344-0
Article Title
Redox-neutral conversion of ubiquitous PV sources to a versatile PO2+ phosphorylation reagent.




