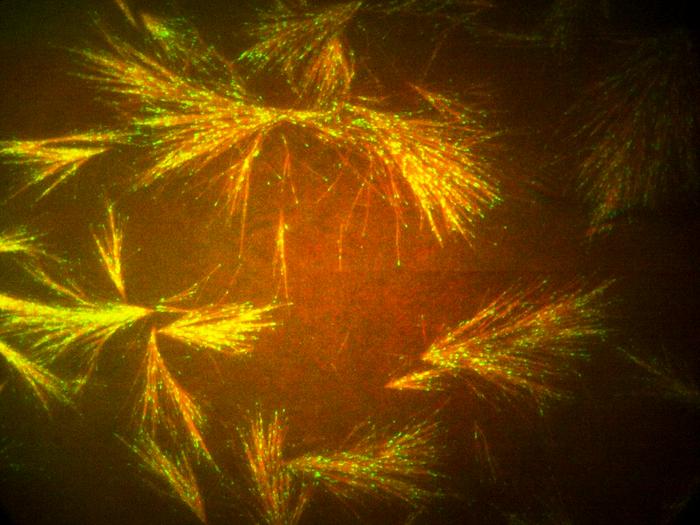
Princeton researchers have learned to harness the gossamer scaffolding that maintains the structure of living cells and used it to develop a nanotechnology platform. The technique eventually could lead to advances in soft robotics, new medicines, and the development of synthetic systems for high-precision biomolecular transport.
Credit: Princeton University
Princeton researchers have learned to harness the gossamer scaffolding that maintains the structure of living cells and used it to develop a nanotechnology platform. The technique eventually could lead to advances in soft robotics, new medicines, and the development of synthetic systems for high-precision biomolecular transport.
In an article published Jan. 17 in the Proceedings of the National Academy of Sciences, the researchers demonstrated a method that allows them to precisely control the growth of bio-polymer networks like those that form part of the cellular skeleton. They were able to build these networks on a microchip, forming a type of circuit operating with chemical, rather than electrical, signals.
Inside cells, tubulin proteins form long, and incredibly thin rods called microtubules. Networks of microtubules grow like tree roots into branching systems that form a primary element of the cytoskeleton that gives cells their shape and enables them to divide.
Besides helping to maintain a cell’s shape, the microtubular scaffolding also works like a molecular railway. Specialized motor proteins carry molecular loads along the microtubule filaments. Slight changes in the microtubules’ molecular makeup act like signposts to adjust the chemical carriers’ courses, sending molecular payloads to their destinations. At Princeton, questions about these intracellular networks led to a collaboration between Sabine Petry, an associate professor of Molecular Biology , and Howard Stone, a professor of mechanical and aerospace engineering who specializes in fluid mechanics.
“The biological systems we were inspired by were axons,” said Meisam Zaferani, one of the lead researchers and a Gilbert S. Omenn, MD. ’61 and Martha A. Darling *70 Fellow in the Bioengineering Institute and Molecular Biology at Princeton. “Axons are long protrusions coming out of a neuron that allow for directed molecular transport.”
In the nervous system, microtubule networks work both as structures connecting nerve cells and as a means for the nervous system to transmit chemical signals that produce sensation. Zaferani said scientists are still working to understand elements of microtubule growth and chemical properties. But he said the research team wanted to know if they could harness the networks for practical applications.
“Engineers and physicists have started to study microtubules as components to build novel materials and technologies,” he said. “There are many mysteries about their fundamental properties, but we know enough to start to think about how we could engineer these systems.”
With co-researcher Ryungeun Song, Zaferani worked to create a system to control the growth of microtubules in the cleanroom labs at the Princeton Materials Institute. Using specialized equipment in micro/nanofabrication and microfluidics, the researchers precisely controlled the growth of the microtubule branches. They were able to adjust the angle and direction of growth and were able to create microstructures in which growth direction of microtubules was regulated. Zaferani said the Materials Institute offered a unique mix of equipment and expertise that would be difficult to find anywhere else.
The researchers plan to follow up by directing chemical cargo along the microtubule branches. The goal is to build a controllable chemical transport system. In a related effort, they are also examining the use of microtubule networks as a tool like micro-tweezers that exert physical force on incredibly tiny objects.
Petry’s research group has long collaborated with Stone, the Donald R. Dixon ’69 and Elizabeth W. Dixon Professor of Mechanical and Aerospace Engineering, at the intersection of biology and fluid dynamics. In 2021, they received a grant from Princeton’s Eric and Wendy Schmidt Transformative Technology Fund. They hired Song, a mechanical engineer who had focused on microfluidics in his graduate work; and Zaferani, a biophysicist who had studied the cues that help mammalian sperm cells navigate toward an egg.
Stone, who frequently collaborates with colleagues in engineering and the natural sciences, said mixing expertise from varied disciplines often leads to remarkable results.
“I find it very interesting to find problems that involve fluid mechanics in other fields,” he said. “Often I find a topic that is poorly understood to the scientists on the other side and poorly understood by myself, and together we work to figure it out.”
The paper, “Building on-chip cytoskeletal circuits via branched microtubule networks,” was published online on Jan. 17 in the Proceedings of the National Academy of Sciences. Support for the project was provided in part by the Princeton University Eric and Wendy Schmidt Transformative Technology Fund.
Journal
Proceedings of the National Academy of Sciences
DOI
10.1073/pnas.2315992121
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Building on-chip cytoskeletal circuits via branched microtubule networks
Article Publication Date
17-Jan-2024




