Osaka, Japan – What if organ damage could be repaired by simply growing a new organ in the lab? Improving researchers’ ability to print live cells on demand into geometrically well-defined, soft complex 3D architectures is essential to such work, as well as for animal-free toxicological testing.
Credit: Shinji Sakai
Osaka, Japan – What if organ damage could be repaired by simply growing a new organ in the lab? Improving researchers’ ability to print live cells on demand into geometrically well-defined, soft complex 3D architectures is essential to such work, as well as for animal-free toxicological testing.
In a study recently published in ACS Biomaterials Science and Engineering, researchers from Osaka University have overcome prior limitations that have hindered cell growth and the geometrical fidelity of bioprinted architectures. This work might help bring 3D-printed cell constructs closer to mimicking biological tissue and organs.
Ever since bioprinting was first reported in 1988 by using a standard inkjet printer, researchers have explored the potential of this layer-by-layer tissue assembly procedure to regrow damaged body parts and test medical hypotheses. Bioprinting is to eject a cell-containing “ink” from a printing nozzle to form 3D structures. It is usually easier to print hard rather than soft structures. However, soft structures are preferable in terms of cell growth in the printed structures. When printing soft structures, doing so in a printing support is effective; however, solidification of ink in the support filled in a vessel can result in its contamination with unwanted substances from the support. Ink solidification into a soft matrix using a printing support without contamination, while retaining cell viability, was the goal of this work.
“In our approach, a 3D printer alternately dispenses the cell-containing ink and a printing support,” explains Takashi Kotani, lead author of the study. “The interesting point is that the support also plays a role in facilitating the solidification of the ink. All that’s necessary for ink solidification is in the support, and after removing the support, the geometry of the soft printed cell structures remains intact.”
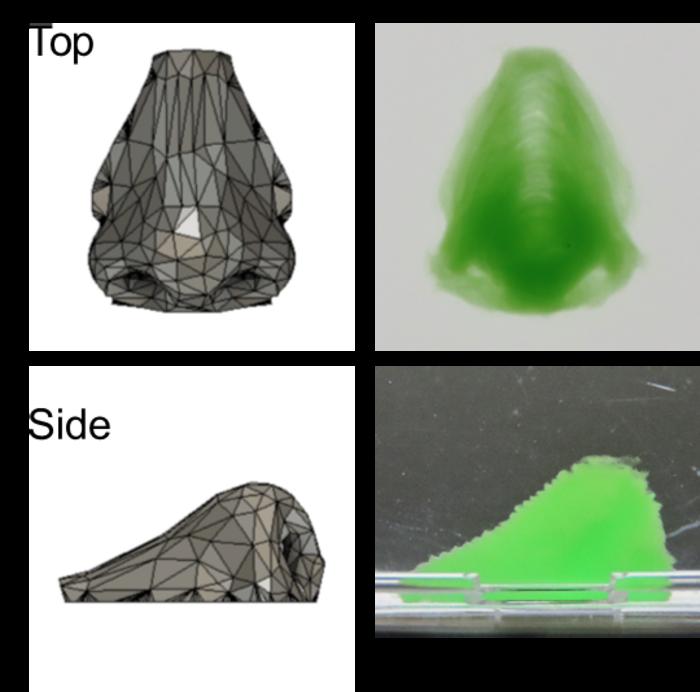
Hydrogen peroxide from the support enabled an enzyme in the ink to initiate gelation of the ink, resulting in a gel-enclosed cell assembly within a few seconds. This rapid gelation prevented contamination of the assembly during formation. After removing the support, straightforward 3D constructs such as inverted trapezium geometries as well as human nose shapes—including bridges, holes, and overhangs—were readily obtained.
“We largely retain mouse fibroblast cell geometry and growth, and the cells remain viable for at least two weeks,” says Shinji Sakai, senior author. “These cells also adhere to and proliferate on our constructs, which highlights our work’s potential in tissue engineering.”
This new technique is an important step forward to engineering human cell assemblies and tissues. Further work might involve further optimizing the ink and support, as well as incorporating blood vessels into the artificial tissue to improve its resemblance to physiological architectures. Regenerative medicine, pharmaceutical toxicology, and other fields will all benefit from this work and further improvements in the precise fidelity of bioprinting.
###
The article, “Horseradish Peroxidase-Mediated Bioprinting via Bioink Gelation by Alternately Extruded Support Material,” was published in ACS Biomaterials Science and Engineering at DOI: https://pubs.acs.org/doi/10.1021/acsbiomaterials.3c00996
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan’s leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan’s most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en
Journal
ACS Biomaterials Science & Engineering
DOI
10.1021/acsbiomaterials.3c00996
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Horseradish Peroxidase-Mediated Bioprinting via Bioink Gelation by Alternately Extruded Support Material
Article Publication Date
26-Sep-2023




