Credit: Tokyo Institute of Technology
Scientists at Tokyo Institute of Technology (Tokyo Tech) demonstrate the first visible-light photoelectrochemical system for water splitting using TiO2 enhanced with an earth-abundant material – cobalt. The proposed approach is simple and represents a stepping stone in the quest to achieve affordable water splitting to produce hydrogen, a clean alternative to fossil fuel.
Photoelectrochemical water splitting, the process by which light energy is used to split water molecules into hydrogen (H2) and oxygen (O2), is a promising approach to obtain pure hydrogen for use as an alternative clean fuel. This process is carried out in electrochemical cells that contain an anode and a cathode submerged in water, which are connected through an external circuit.
At the anode, water oxidation occurs, whereby O2 is produced by drawing energy from light waves. These waves transfer energy to the electrons of the anode material, allowing them to move through the external circuit to reach the cathode. Here, the received electrons and the cathode material cause H2 to form.
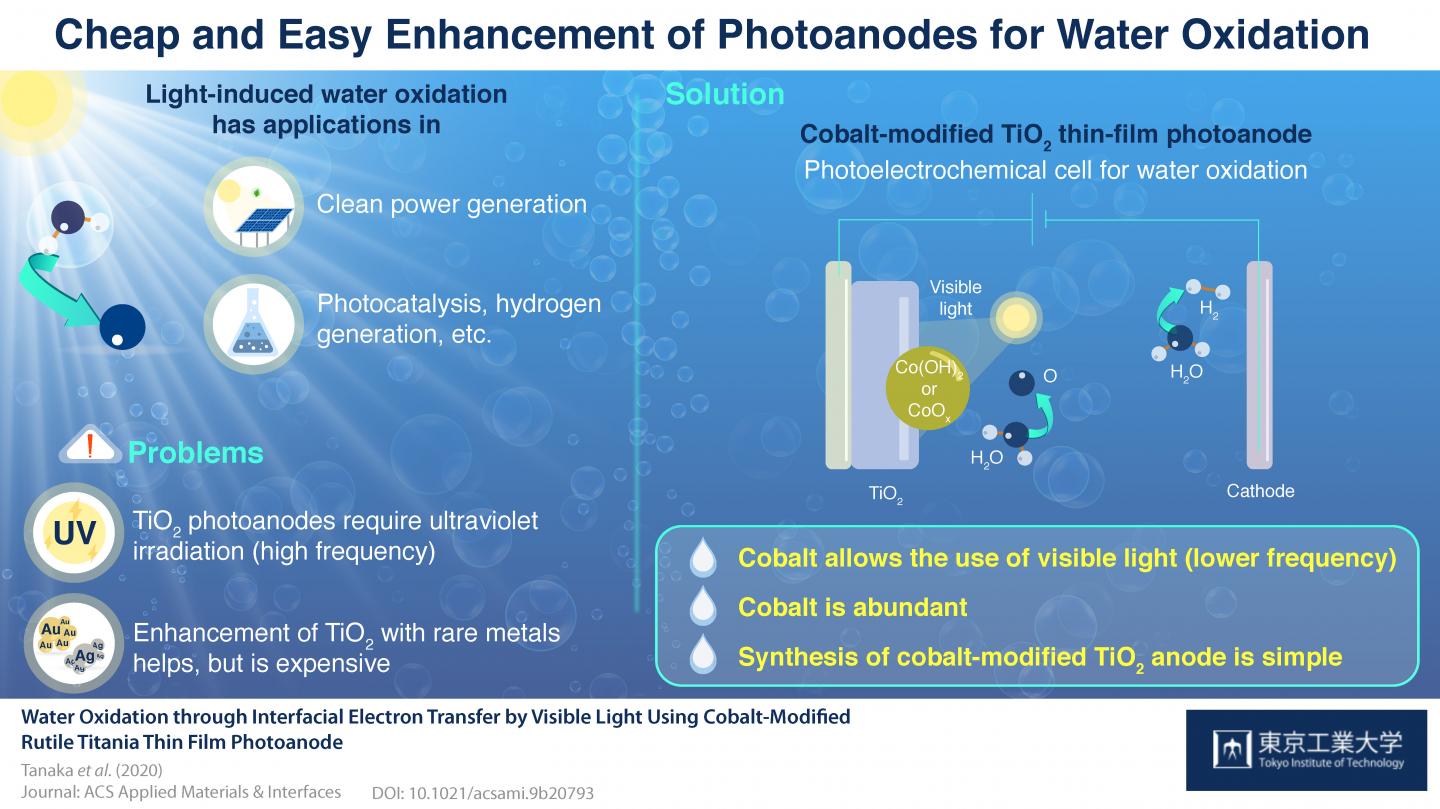
To date, it has been difficult to find photoelectrochemical systems that carry this process efficiently due to various reasons. Titanium dioxide (TiO2), a well-known and widely used photoanode material, can only absorb energy from light in the ultraviolet region; that is, high-energy light. Because it would be preferable to leverage the energy from longer-wavelength light, TiO2 can be mixed with noble metals (such as gold or silver) to sensitize it to visible light, but this would be expensive in large-scale applications.
To find a solution to this problem, a research team from Tokyo Tech created the first visible-light photoanode made of TiO2 enhanced with an earth-abundant material – cobalt. Their study published in ACS Applied Materials & Interfaces explains the surprisingly simple process of photoanode fabrication; thin TiO2 films are grown onto a substrate through a standard procedure and then cobalt is introduced by immersing them into an aqueous cobalt nitrate solution. “This study demonstrates that a visible light-driven photoelectrochemical cell for water oxidation can be constructed through the use of earth-abundant metals without the need for complicated preparation procedures,” remarks Prof. Kazuhiko Maeda, who led the research.
Through multiple types of spectrometry analyses and scanning electron microscopy, the researchers identified the specific composition and structure of the cobalt-modified surface of the TiO2 photoanode to understand how cobalt allows the material to absorb visible light to mobilize electrons and cause water oxidation. It turns out that cobalt domains not only capture visible light and transfer charges (electrons) at the TiO2 interface, but also serve as catalytic sites that facilitate water oxidation. Moreover, the researchers found that the structure of the base TiO2 thin film affects the performance of the final modified photoanode, presumably by allowing for a better or worse accommodation of cobalt atoms. The structure of the TiO2 film can be easily tuned by adjusting fabrication parameters, which allowed the team to carry out multiple tests to gain insight on this phenomenon.
More work still needs to be done, as it will be necessary to further optimize the design of the photoanode to improve the charge transfer process that occurs between the cobalt atoms and the TiO2 substrate to achieve higher water oxidation rates. Nevertheless, a major advantage of the proposed water oxidation system is that it is non-sacrificial; in other words, the materials employed do not rely on energy-rich oxidants and/or reductants (i.e., sacrificial reagents). “So far, cobalt-sensitized water photooxidation systems had been comprised of powder-based photocatalysis, which work only in the presence of a sacrificial electron acceptor. Therefore, the present study also demonstrates sacrificial reagent-free visible-light water splitting using a cobalt-sensitized semiconductor material (TiO2),” concludes Prof. Maeda. This study will hopefully serve as a stepping stone for all those trying to achieve affordable water splitting to ensure a greener future.
###
Media Contact
Emiko Kawaguchi
[email protected]
81-357-342-975
Related Journal Article
http://dx.