Credit: Takuya Shimajiri, Takanori Suzuki, Yusuke Ishigaki, Angewandte Chemie International Edition, September 30, 2020
A Hokkaido University research group has successfully demonstrated that carbon-carbon (C-C) covalent bonds expand and contract flexibly in response to light and heat. This unexpected flexibility of C-C bonds could confer new properties to organic compounds.
Rigid and robust, C-C covalent bonds are the most basic structure in organic and biological compounds. Understanding their nature is essential to improving our knowledge of chemical phenomena.
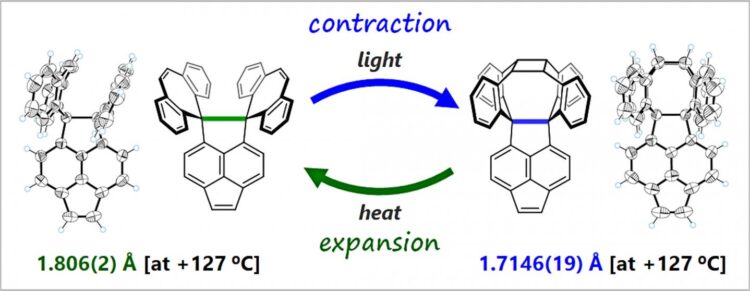
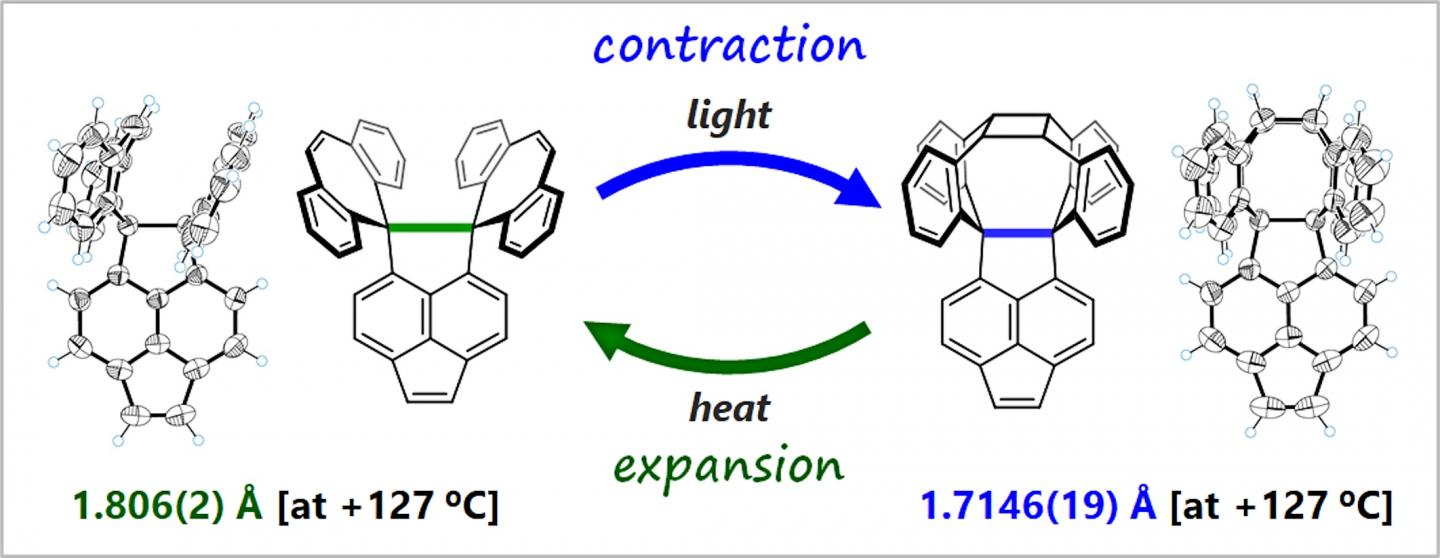
Usually, the C-C bond length is almost constant. The researchers, however, conducted this study on the premise that extremely elongated C-C bonds are weak, and so can expand or contract in response to external stimuli. The group designed and synthesized compounds that cyclize to form cage-like structures when exposed to light. They investigated how the structural transformation influences the length of C-C bonds at compounds’ cores.
The researchers found that the C-C single bonds at the core contract flexibly during photocyclization. They also found that the cyclization can be reversed by heating, and the C-C bonds expand as the compounds return to the original state. Using single crystals of the compounds as analogs made it possible for the researchers to directly observe their flexibility and easily elucidate their structure in detail.
This is the first time the process of expansion and contraction of C-C bonds has been directly observed. The scientists concluded that this is a new phenomenon, in which C-C bonds obtained flexibility when they were elongated to the limit, decreasing the bonding energy. Furthermore, they showed that the oxidation potential of the compound changed by more than 1 volt due to the reversible expansion and contraction of the extremely elongated C-C bond, suggesting a new property related to the bond’s flexibility.
The researchers say that synthesizing compounds with even longer bonds may lead to more functions through unique responses or major changes in their properties. This challenging research, aimed at breaking the record for the length of the C-C single bond, plays a role in developing materials that can be activated/deactivated by a novel response mode.
The researchers are Takuya Shimajiri, Professor Takanori Suzuki and Assistant Professor Yusuke Ishigaki of Hokkaido University’s Department of Chemistry. The results of their study were published in Angewandte Chemie International Edition on September 30, 2020. This work follows their study in 2018, in which the group synthesized an organic compound with a record C-C bond length of more than 0.18 nanometers, compared to the standard 0.154 nanometers.
###
Media Contact
Sohail Keegan Pinto
[email protected]
Original Source
https:/
Related Journal Article
http://dx.