Credit: Osaka University
Osaka, Japan – Before cell division when a parent cell divides into two daughter cells, each with an identical set of genetic information, replicated chromosomes become condensed and align along the spindle in the middle of the cell. This is the metaphase stage of mitosis. Chromosomes are made up of strands of DNA wrapped around histones, forming nucleosomes that resemble beads on a string. The chromosome structure is maintained by histone changes, chromosome scaffold proteins, and positively charged ions (cations). However, removal of scaffold proteins has no effect on chromosome condensation, suggesting that another factor causes the organization of metaphase chromosomes. Now, work led by Osaka University has revealed that calcium ions (Ca2+) play a crucial role in chromatin fiber compaction. The study was reported in Scientific Reports.
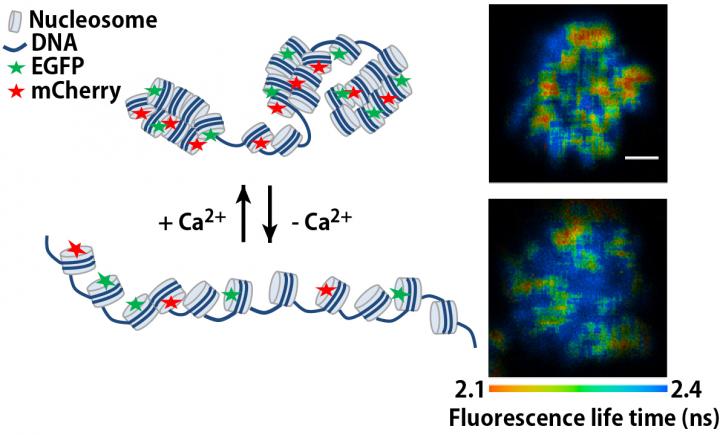
Calcium is a universal second messenger with many functions, including progression of the cell cycle. During mitosis, both Ca2+ and magnesium ions (Mg2+) are released from storage organelles to bind chromatin, though 6-8-fold more Ca2+ is bound than Mg2+ suggesting that it has a more important role. A research team led by Hideaki Takata, Kiichi Fukui and Rinyaporn Phengchat at Osaka University first confirmed that Ca2+ is necessary to prevent chromosome misalignment during mitosis, then used an imaging assessment of molecular interactions based on fluorescence decay (FLIM-FRET) to show that chromosomes were less compact in the absence of intracellular Ca2+.
This same imaging process also revealed that changes in Ca2+ levels of living HeLa cells altered the extent of chromosome compaction, with the re-addition of Ca2+ causing chromosome compaction. Ca2+ therefore appears to control the transition between condensation and decondensation. "Although previous studies have demonstrated nucleosome packaging using isolated chromatin" study first author Rinyaporn Phengchat says, "such findings should be confirmed in vivo as we have done using the sensitive FLIM-FRET technique which provides a high level of spatial resolution."
The team then used scanning electron microscopy to visualize chromosome structures at very high resolution. "Ca2+ has a concentration-dependent effect on chromosome structure," corresponding author Hideaki Takata explains, "causing chromosomes to change from expanded fibrous structures to compact globular structures with increasing levels of Ca2+."
The researchers propose that, in the absence of Ca2+, the negatively charged DNA is less well neutralized, preventing it from condensing and delaying entry into prometaphase of the cell cycle. "We think that a lack of Ca2+ disturbs the organization of chromosomes at the spindle, causing them to misalign," corresponding author Kiichi Fukui says. "Conversely, the normal rising levels of Ca2+ during mitosis promote chromosome condensation after breakdown of the nuclear envelope."
###
Media Contact
Saori Obayashi
[email protected]
81-661-055-886
@osaka_univ_e
http://www.osaka-u.ac.jp/en
############
Story Source: Materials provided by Scienmag





