There’s been lots of talk about capturing carbon dioxide from the air, but what about removing it from the ocean?
Credit: University of Pittsburgh
There’s been lots of talk about capturing carbon dioxide from the air, but what about removing it from the ocean?
About 25 percent of carbon dioxide emitted today is eventually trapped in earth’s oceans and is causing acidification and damage to important ecosystems such as coral reefs. Finding a way to capture carbon dioxide in the ocean would not only help to mitigate climate change, but also preserve marine life.
Capturing carbon dioxide from the ocean – called direct ocean capture – has gained traction in recent years as a complementary approach to direct air capture.
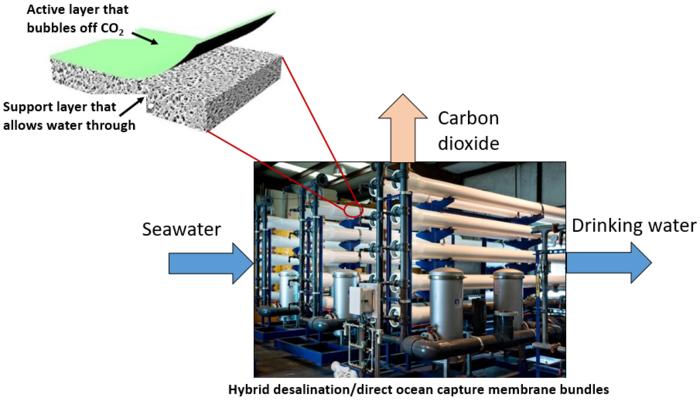
Katherine Hornbostel, the principal investigator on a multi-institution proposal and assistant professor of mechanical engineering and materials science at the University of Pittsburgh Swanson School of Engineering, recently received a $1.4 million award from the National Oceanic and Atmospheric Administration and Office of Naval Research to develop new membranes that can simultaneously perform desalination and direct ocean capture. The team includes members from Arizona State University, University of California-Irvine, and the National Renewable Energy Laboratory (NREL).
“Removing carbon dioxide from the ocean is tricky, but leveraging a similar existing technology through desalination could make it much more feasible,” Hornbostel explained. “We’re looking for an alternative that saves both energy and costs.”
Bubble off, CO2!
Desalination – the process of removing salt from ocean water – has become a sought-after method to provide fresh drinking water to regions of the world living with contaminated water or little access to fresh drinking water. How could it possibly play a role in direct ocean capture?
The most common method for direct ocean capture relies on an electrochemical cell. Though effective in removing carbon dioxide, using electrochemical cells is energy-intensive and expensive. Hornbostel – who is well-versed in carbon capture technologies – and her team are looking to prove that coating desalination membranes with special chemical groups can effectively “bubble off”– or release – carbon dioxide gas for capture and storage or reuse, similar to when a carbonated beverage is opened.
“Using our approach should require far less electricity to strip carbon dioxide from seawater compared to more conventional methods that electrochemically split all of the incoming seawater into basic and acidic streams,” Hornbostel said.
But first, the team will have to determine what membrane is best for the bubbling off to work.
More energy or less CO2?
There are two membranes that may be the answer, but they present their own unique problems.
The team will study both reverse osmosis (RO) and nanofiltration (NF) membranes. The NF membranes have a lower energy input, but a lower carbon dioxide removal rate. The RO membranes have a high carbon dioxide removal rate, but a high energy input.
“We’re planning to run a technoeconomic assessment that will allow us to compare these two options and determine which one is more scalable outside of a lab setting and at an actual desalination plant,” Hornbostel said.
The two-year project, “Coupling Desalination with Novel Marine Carbon Dioxide Removal Membranes,” will also be led by the following co-Principal Investigators:
- Professor Matthew Green, Arizona State University
- Professor Jenny Yang, UC Irvine
- Dr. Abhishek Roy, National Renewable Energy Laboratory
- Dr. Mou Paul, National Renewable Energy Laboratory




