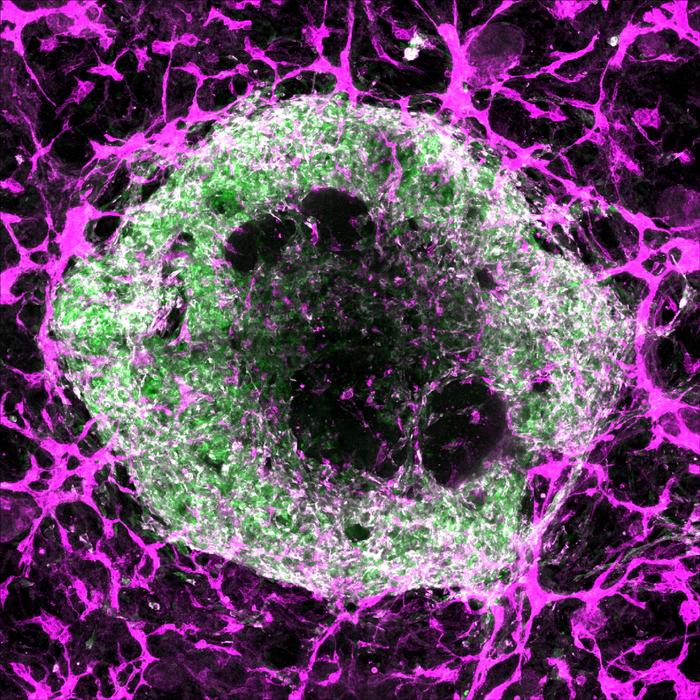
For over a decade, organoids—tiny, three-dimensional clusters of cells that emulate the structure and function of human organs—have revolutionized biomedical research by providing miniature, simplified models that enhance our understanding of development, disease, and therapeutic interventions. Brain organoids have illuminated aspects of neurodevelopmental disorders, intestinal organoids have modeled diseases like celiac, and lung organoids have been pivotal in studying viral infections such as SARS-CoV-2. Remarkably, heart organoids have even ventured beyond Earth, sent to orbit in space to assess how microgravity affects cardiac muscle. Despite these advances, organoids face a fundamental constraint: they cannot grow beyond a few millimeters in diameter, roughly the size of a sesame seed, due to the absence of a vital blood vessel network capable of sustaining larger tissue masses.
Addressing this challenge, a pioneering study published in Science on June 5, 2025, led by Stanford Medicine researchers including Oscar Abilez, MD, PhD, and Huaxiao (Adam) Yang, introduces a breakthrough approach to cultivating vascularized heart and liver organoids. These organoids incorporate tiny blood vessels formed by endothelial and smooth muscle cells, achieving an integrated vascular network capable of sustaining larger and more complex tissue models. This advance holds transformative potential for organoid technology, offering not only increased size and durability but also enhanced cellular diversity and maturity, bringing researchers closer to replicating physiological organ environments in vitro.
.adsslot_eg13lyinKJ{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_eg13lyinKJ{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_eg13lyinKJ{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Central to this achievement was a meticulous optimization of the biochemical environment—essentially, the “recipe” of growth factors and signaling molecules guiding pluripotent stem cells through differentiation into specialized cardiac cell types. Previous attempts to vascularize organoids often yielded inconsistent proportions of cardiomyocytes, endothelial cells, and smooth muscle cells, crucial for establishing functional vascular systems. Some researchers employed engineering strategies like separately growing vascular cells or 3D bioprinting vessel networks, but these methods failed to reproduce the authentic branched capillary structures that characterize living organs.
In this study, the research team developed and tested 34 distinct differentiation protocols, systematically varying the timing, dosage, and combination of growth factors to mimic embryonic heart development signals. They also genetically tagged the stem cells with fluorescent markers that light up when the cells mature into cardiomyocytes, endothelial cells, or smooth muscle cells, allowing precise visualization and quantification of the resulting cell populations. Among these experimental conditions, one particular protocol—referred to as “condition 32”—stood out by reliably producing cardiac organoids rich with all three critical cell types, arranged into complex, organized structures replicating the heart’s cellular composition.
To further characterize the cellular complexity of these vascularized organoids, the researchers employed single-cell RNA sequencing, a powerful technique that profiles gene expression in individual cells. The analysis revealed that the organoids contained an unexpectedly rich diversity of 15 to 17 distinct cardiac cell types, closely aligning with the cellular heterogeneity found in a six-week-old embryonic human heart, which naturally comprises approximately 16 cell types. This degree of complexity surpasses prior organoid models and approaches the cellular diversity seen in native organ tissue, heralding improved physiological relevance for disease modeling and drug testing.
Intriguingly, the success of the optimized protocol seems to mirror the biochemical milieu of early embryonic heart development, precisely the stage when cellular diversification and vascularization begin in vivo. By recapitulating these developmental cues, the organoids serve as windows into the earliest phases of human organogenesis—a largely inaccessible period due to ethical and technical constraints. This feature opens novel avenues for investigating how embryonic tissues form, how genetic or environmental factors might perturb development, and how drugs might affect early organ growth with potential implications for pregnancy and fetal health.
Demonstrating the organoids’ utility in pharmacological research, the team exposed them to fentanyl, a potent opioid widely abused yet still poorly understood regarding its effects during development. Remarkably, the vascularized cardiac organoids responded by increasing blood vessel formation, indicating that drug exposure modulates vascular development in the heart. While the clinical implications for newborns remain to be fully elucidated, these insights could influence the understanding of how in utero exposure to opioids might impact cardiac development and postnatal health.
Extending beyond the heart, the researchers applied similar vascularization strategies to liver organoids by combining established protocols for directing pluripotent stem cells toward hepatic lineages with those for vascular cell differentiation. These liver organoids also formed robust vascular networks, substantiating the versatility and broad applicability of the approach across multiple organ systems. This versatility holds promise for generating more physiologically relevant organoid models for a range of tissues that depend on dense vascularization, including kidneys, pancreas, and lungs.
Looking ahead, the research team aims to culture these vascularized organoids for extended periods to assess their growth potential and maturation, striving to develop organoids not only larger in size but also functionally mature and capable of faithfully modeling adult tissues. They are also exploring recipe refinements to incorporate additional cell types critical for proper organ function—such as immune cells and blood cells—toward more faithfully recapitulating the cellular mosaic of adult organs and enabling realistic disease modeling, including chronic conditions and immune responses.
Beyond modeling and drug testing, the study sets the stage for pioneering regenerative medicine applications. Current clinical trials led by Joseph Wu, MD, PhD, involve injecting lab-grown cardiomyocytes into patients with cardiac dysfunction; however, actual heart tissue comprises a complex interplay of multiple cell types embedded in a vascularized matrix. Implanting vascularized cardiac organoids derived from a patient’s own stem cells could improve integration with host tissue, enhance survival and function, and potentially revolutionize the treatment of heart disease by replacing lost or damaged myocardium with engineered living tissue.
These advancements illustrate that the future of organoid technology lies in overcoming the vascularization bottleneck, allowing researchers to grow functional, mature mini-organs fit for translational and therapeutic applications. As Oscar Abilez reflects, “If organoids have a vascular system, they could connect with the host vasculature, giving them a better chance to survive.” This vision portends a new era where patient-specific, vascularized organoids not only revolutionize personalized medicine but could also serve as living grafts to restore organ function, thereby transforming the landscape of biomedical science and healthcare.
Subject of Research: Cells
Article Title: (Not explicitly provided in the source content)
News Publication Date: 5-Jun-2025
Web References: http://dx.doi.org/10.1126/science.adu9375
References: (Not explicitly provided in the source content)
Image Credits: Oscar Abilez/Stanford Medicine
Keywords: Organ cultures, Cardiology
Tags: 3D cell culture modelsavascular organoid limitationsenhancing organoid vascularizationfuture of organoid technologyheart organoids in space researchintestinal organoids for celiac diseaselung organoids and viral infectionsmetabolic demands of organoidsneurodevelopmental disorder researchorganoid applications in medicineorganoid development breakthroughsorganoid modeling diseases