In a groundbreaking study from Japan, researchers have successfully developed a method to generate lung cells akin to alveolar epithelial type 2 (AT2) cells using mouse embryonic fibroblasts, bypassing the need for stem cell technology altogether. This innovative approach, completed in a swift timeframe of just 7 to 10 days, marks a significant leap forward compared to the traditional stem cell differentiation methods that typically span approximately one month. The implications of this research are profound, especially for patients suffering from severe respiratory diseases, including interstitial pneumonia and chronic obstructive pulmonary disease, which currently have no effective treatment options.
AT2 cells play a critical role in maintaining lung homeostasis, which involves the secretion of surfactant and acting as progenitor cells that facilitate alveolar repair. In individuals afflicted with severe lung conditions, the number of functional AT2 cells can be severely diminished, underscoring the urgent need for strategies that focus on their regeneration. The researchers published this study in the prestigious journal “npj Regenerative Medicine,” highlighting the potential of their findings to transform therapeutic approaches in lung disease treatment.
Professor Makoto Ishii of Nagoya University Graduate School of Medicine, along with his colleagues, has emphasized the disadvantages associated with induced pluripotent stem cell technology, first introduced in 2006. Although this method allows for the generation of AT2 cells within a one-month timeframe, it is fraught with challenges stemming from high costs, risk of tumor formation, and complications associated with immune rejection. To address these issues, the research team shifted their focus toward direct reprogramming, a technique that not only shortens the timeline but also minimizes tumor risk, enhancing the potential for autologous transplantation.
.adsslot_1tFTBiWboZ{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_1tFTBiWboZ{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_1tFTBiWboZ{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
The team embarked on their journey by selecting 14 candidate genes that are crucial for lung development. Preliminary investigations led them to discover that the expression levels of the AT2 cell marker surfactant protein-C (Sftpc) could provide insights into which gene combinations would yield the highest reprogramming efficiency. At the heart of their findings lay a powerful combination of four genes—Nkx2-1, Foxa1, Foxa2, and Gata6—that proved to be exceptionally effective for reprogramming fibroblasts into AT2-like cells.
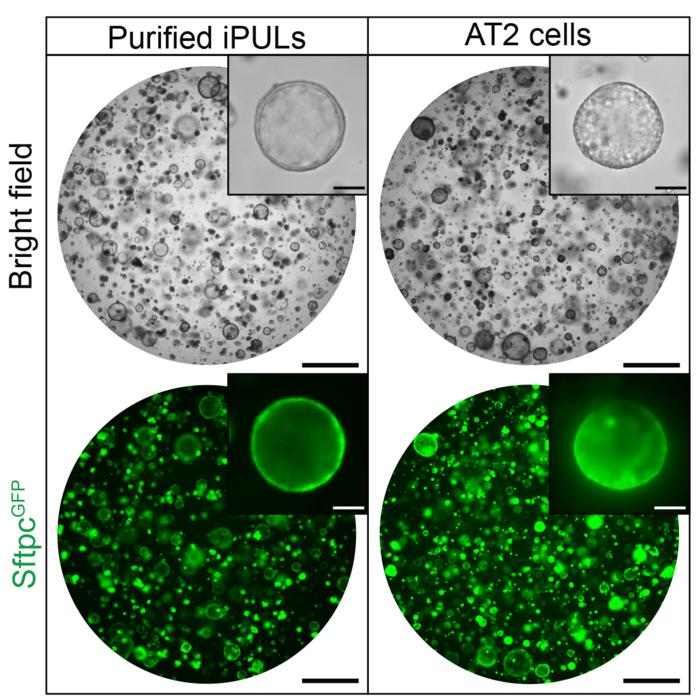
In the experimental setup, these four genes were introduced into a three-dimensional culture system derived from mouse embryonic fibroblasts, visualized using a green fluorescent protein (GFP) marker activated by Sftpc. Astonishingly, within the 7 to 10-day timeframe, approximately 4% of the cultured cells expressed Sftpc and GFP, indicating a remarkable success in inducing AT2-like cells, which have been termed induced pulmonary epithelial-like cells (iPULs).
After isolating GFP-positive cells through flow cytometry, the researchers conducted comprehensive analyses of the iPULs. Their observations confirmed the presence of lamellar body-like structures within these cells, a hallmark feature indicative of normal AT2 cells. Transcriptomic profiling further revealed that the gene expression patterns of the iPULs were predominantly aligned with those of native AT2 cells, effectively validating the effectiveness of their reprogramming approach.
Following this success, the team advanced to test the therapeutic efficacy of their iPULs by transplanting purified cells into the lungs of mice suffering from interstitial pneumonia. Remarkably, 42 days post-transplantation, the iPULs demonstrated successful engraftment within the alveolar regions, with a significant portion of the cells transitioning into alveolar epithelial type 1 (AT1)-like cells. These cells are vital for the regeneration of lung tissue, further establishing the therapeutic potential of this innovative technology.
Professor Ishii concluded the study by emphasizing the next phase of research: exploring the practical application of this promising technology using human cells. The ultimate vision is to cultivate a safe regenerative therapy utilizing a patient’s own fibroblasts, enhancing treatment outcomes while minimizing risks associated with immunogenicity and tumorigenesis. This pioneering work not only sheds light on a novel pathway for lung cell regeneration but also opens avenues for future research dedicated to addressing chronic respiratory diseases, ultimately improving quality of life for countless patients.
The implications of this research are vast, as it may lead to new cell-based therapies that harness the power of a patient’s own cells to create personalized treatments. The team is optimistic about further investigations aimed at translating their findings into clinical applications, potentially reshaping therapeutic strategies for respiratory ailments. As the study demonstrates a new horizon for regenerative medicine, it sparks hope for innovative treatments that could combat conditions previously deemed unmanageable.
With ongoing funding from a suite of grants provided by JSPS KAKENHI and AMED, which support the advancement of scientific research, the researchers are poised to continue their exploration into the world of cellular reprogramming. This study stands as a remarkable testament to the potential within regenerative medicine, as the scientific community eagerly anticipates further developments in this cutting-edge field.
The importance of finding effective solutions for chronic respiratory diseases cannot be overstated, given the increasing prevalence of such conditions worldwide. As advancements like those made by Professor Ishii and his team progress, they represent critical milestones toward ultimately developing innovative therapies that could significantly alter the treatment landscape for patients battling debilitating lung diseases.
In conclusion, the ability to generate AT2-like cells from fibroblasts with speed and reduced risk holds transformative potential for the field of regenerative medicine. This study not only lays the groundwork for future exploration into human applications but also emphasizes the importance of rethinking traditional approaches in cellular therapy. With enthusiasm and dedication to advancing medical science, Professor Ishii and his team are making strides that may one day lead to practical solutions for some of the most challenging health issues of our time.
Subject of Research: Animals
Article Title: Direct reprogramming of mouse fibroblasts into self-renewable alveolar epithelial-like cells
News Publication Date: 23-Jun-2025
Web References: npj Regenerative Medicine
References: DOI 10.1038/s41536-025-00411-4
Image Credits: Credit: Makoto Ishii
Keywords
Regenerative medicine, Medical technology, Respiratory disorders, Pneumonia, Chronic obstructive pulmonary disease, Health and medicine.
Tags: alveolar epithelial type 2 cellschronic obstructive pulmonary disease innovationsinterstitial pneumonia researchlung cell regenerationlung homeostasis maintenanceMakoto Ishii researchmouse fibroblast conversionrapid cell differentiation methodsregenerative medicine advancementsrespiratory disease treatmentsstem cell technology alternativestherapeutic approaches for lung diseases