In a groundbreaking advancement in the field of biomedical engineering, researchers at Carnegie Mellon University have unveiled a revolutionary 3D bioprinting technique that harnesses the intrinsic properties of collagen to create fully biologic tissue models. The innovative approach, known as Freeform Reversible Embedding of Suspended Hydrogels (FRESH), has allowed scientists to fabricate microphysiologic systems that closely mimic human physiology. By leveraging collagen, the most abundant protein in the body, this cutting-edge technology promises to transform the landscape of disease modeling and tissue engineering, particularly for conditions such as Type 1 diabetes.
Collagen traditionally serves as a fundamental structural protein in human tissues, supporting everything from skin integrity to organ function. However, understanding its full potential requires a shift away from conventional tissue engineering methods that rely heavily on synthetic materials. Past approaches have utilized plastics and silicone rubbers for creating organ-on-chip models and microfluidic systems, which, while innovative, cannot perfectly replicate the biological environment in which human cells thrive. Consequently, this limitation has stifled the development of truly functional tissue models for research and therapeutic applications.
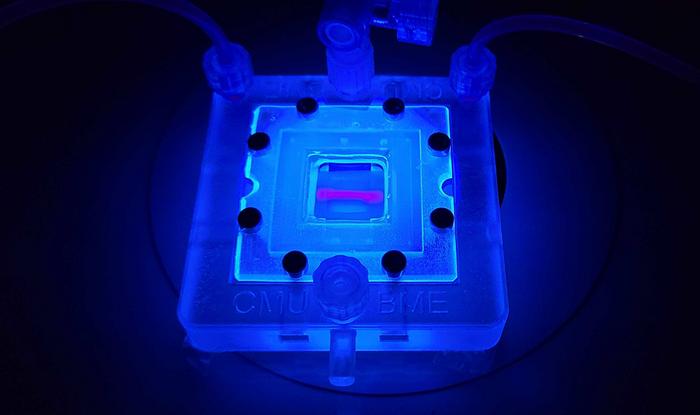
With the introduction of FRESH bioprinting technology, Carnegie Mellon’s Feinberg lab has embarked on a mission to construct entirely collagen-based tissue scaffolds that integrate living cells with unprecedented fidelity and resolution. This pioneering work was documented in the journal Science Advances, where the authors detailed their successful creation of complex vascularized tissues that could potentially serve as functional substitutes for damaged pancreatic tissues in diabetic patients. The ability to print structures with rapidly interchangeable designs marks a significant leap forward in both bioprinting and regenerative medicine.
The key advantage of this methodology lies in its biologic compatibility, enabling the systematic assembly of organ-like structures that can support cell growth and activity; thereby offering an avenue for researchers to observe the biological processes of diseases in a controlled environment. Adam Feinberg, a professor at Carnegie Mellon University deeply involved in this research, stated that the achievement of building fully biologic microfluidic systems from collagen, cells, and proteins has the potential to accelerate our understanding of pathophysiological mechanisms and therapeutic interventions for ailments such as diabetes.
Advancements in FRESH bioprinting technology are not merely academic, as highlighted by Daniel Shiwarski, an assistant professor of bioengineering at the University of Pittsburgh and key contributor to this research. By refining the bioprinting process to a single-step fabrication technique, they accomplished the remarkable feat of producing high-resolution, internally perfusable collagen scaffolds. These scaffolds feature intricate fluidic channels that mimic human vascular systems, providing the necessary conditions for blood supply and nutrient delivery, which are crucial for the survival and function of implanted tissues.
The team’s innovative approach allows for the development of centimeter-scale pancreatic-like tissue constructs that exhibit glucose-stimulated insulin release, outperforming existing organoid-based therapy methods. The implications of this technological breakthrough for treating Type 1 diabetes are monumental, offering the promise of transforming the standard of care for thousands of individuals suffering from this autoimmune disorder.
FluidForm Bio, a startup spun out from the work conducted at Carnegie Mellon, is actively commercializing this state-of-the-art technology. Under the leadership of Dr. Andrew Hudson, the team has already demonstrated in animal models the exciting capability of their collagen-based tissue systems to restore normal insulin production, an achievement that positions them to enter clinical trials within the next few years. Such advancements signal the potential for scalable therapies that may one day replace insulin injections for those living with diabetes.
The implications of the research extend beyond just treatment solutions. Feinberg emphasizes that the importance of collaborative, interdisciplinary research cannot be overstated. Involvement from specialists across various fields—ranging from molecular biology to materials science—contributes not only to technological advancement but also broadens the societal impact through innovations that address pressing health challenges.
As the landscape of tissue engineering evolves with innovations like the FRESH bioprinting technique, researchers are increasingly tasked with determining the next logical steps in application. With advancements in computational modeling and machine learning, scientists aim to better understand and design biologically relevant tissue constructs that not only replicate the architecture of natural organs but also fulfill specific functional roles when implanted into patients.
The commitment to open-source design is a cornerstone of this research, promoting accessibility and adaptability across the global scientific community. Feinberg envisions a future where labs worldwide can adopt these technologies, amplifying their application to a variety of diseases, while fostering a platform for building increasingly intricate and sophisticated tissue systems. This accessibility could catalyze the rapid development of new treatment avenues.
As researchers continue to push the boundaries of how we understand and utilize biomaterials, the potential for FRESH bioprinting and collagen-based architectures in regenerative medicine appears boundless. The ability to fabricate biologically relevant tissues could revolutionize not just diabetes management but also myriad applications in regenerative therapies and drug testing.
The extraordinary advancements described here mark a significant milestone in the intersection of bioengineering and medicine. The collaborative efforts and innovations coming out of the Carnegie Mellon University Feinberg lab illuminate a promising path toward the future of bioprinting technologies, where the integration and customization of tissue systems embody the potential for unimaginable medical breakthroughs that could change the fabric of health care.
Subject of Research:
Strong advancements in 3D bioprinting technology utilizing collagen for tissue models.
Article Title:
3D Bioprinting of collagen-based high-resolution internally perfusable scaffolds for engineering fully biologic tissue systems.
News Publication Date:
23-Apr-2025
Web References:
Science Advances Article
References:
Science Advances
Image Credits:
Daniel Shiwarski, assistant professor of bioengineering at the University of Pittsburgh and prior postdoctoral fellow in the Feinberg lab.
Keywords
Biomedicine, 3D bioprinting, collagen, tissue engineering, Type 1 diabetes, regenerative medicine, microfluidics, vascularized tissues, insulin production, bioengineering, organ-on-chip, advanced fabrication.
Tags: 3D bioprinting technologybiomedical engineering advancementscollagen-based tissue scaffoldsdisease modeling techniquesFreeform Reversible Embedding of Suspended Hydrogelshuman physiology replicationinnovative tissue engineering methodsmicrophysiologic systemsorgan-on-chip developmenttherapeutic applications of bioprintingType 1 diabetes researchvascularized tissue engineering