A team of chemists from the University of Vienna, led by Nuno Maulide, has achieved a significant breakthrough in the field of chemical synthesis, developing a novel method for manipulating carbon-hydrogen bonds. This groundbreaking discovery provides new insights into the molecular interactions of positively charged carbon atoms. By selectively targeting a specific C–H bond, they open doors to synthetic pathways that were previously closed – with potential applications in medicine. The study was recently published in the prestigious journal Science.
Credit: Maulide Group
A team of chemists from the University of Vienna, led by Nuno Maulide, has achieved a significant breakthrough in the field of chemical synthesis, developing a novel method for manipulating carbon-hydrogen bonds. This groundbreaking discovery provides new insights into the molecular interactions of positively charged carbon atoms. By selectively targeting a specific C–H bond, they open doors to synthetic pathways that were previously closed – with potential applications in medicine. The study was recently published in the prestigious journal Science.
Living organisms, including humans, owe their complexity primarily to molecules consisting mainly of carbon, hydrogen, nitrogen, and oxygen. These building blocks form the basis of countless substances essential for daily life, including medications. When chemists embark on synthesizing a new drug, they manipulate molecules through a series of chemical reactions to create compounds with unique properties and structures.
This process involves breaking and forming bonds between atoms. Some bonds, such as those between carbon and hydrogen (C–H bonds), are particularly strong and require considerable energy to break, while others can be more easily modified. Whereas an organic compound typically contains dozens of C–H bonds, chemists traditionally had to resort to manipulating other, weaker bonds. Such bonds are far less common and often need to be introduced in additional synthetic steps, making such approaches costly – thus, more efficient and sustainable synthetic methods are sought after.
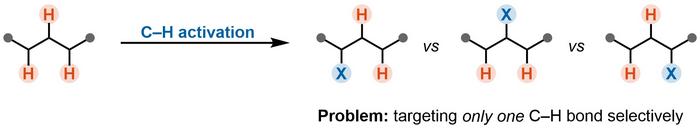
C–H Activation as a New Approach
The concept of C–H activation is a revolutionary approach enabling the direct manipulation of strong C–H bonds. This breakthrough not only enhances the efficiency of synthetic processes but can also often reduce their environmental impact and provide more sustainable paths for drug discovery.
A key challenge is the precise manipulation of a specific C–H bond within a molecule containing many different C–H bonds. This obstacle, known as the “selectivity problem,” often hinders the broader application of established C–H activation reactions (Figure 1).
Targeting a Specific C–H Bond
Researchers at the University of Vienna led by Nuno Maulide have now developed a new C–H activation reaction that addresses the selectivity problem and enables the synthesis of complex carbon-based molecules. By selectively targeting a specific C–H bond with remarkable precision, they open doors to synthetic pathways that were previously closed.
The Maulide group focuses on so-called “carbocations” (i.e., molecules containing a positively charged carbon atom) as key intermediates. “Traditionally, carbocations react by eliminating a hydrogen atom adjacent to the carbon atom, forming a carbon-carbon double bond in the product,” explains Nuno Maulide (Figure 2A). “Products with double bonds – called alkenes – can be extremely useful. However, sometimes a single bond instead of a double bond is desired,” continues the multiple ERC awardee. “We have discovered that in certain cases, reactivity can take a new direction. This leads to a phenomenon called ‘remote elimination,’ resulting in the formation of a new carbon-carbon single bond – a phenomenon that has not been investigated before,” explain Phillip Grant and Milos Vavrík, first authors of the study (Figure 2B).
The researchers demonstrated this new reactivity by synthesizing decalins, a building block for many pharmaceuticals. “Decalins are a class of cyclic carbon-based molecules found in many biologically active compounds. We can now produce these molecules in a much more efficient manner, potentially contributing to the development of new and more effective drugs,” concludes Nuno Maulide, the 2019 Austrian Scientist of the Year.
Journal
Science
DOI
10.1126/science.adi8997
Article Title
Remote proton elimination: C–H activation enabled by distal acidification
Article Publication Date
16-May-2024




