DURHAM, N.C. – Magnetic resonance imaging (MRI) is how we visualize soft, watery tissue that is hard to image with X-rays. But while an MRI provides good enough resolution to spot a brain tumor, it needs to be a lot sharper to visualize microscopic details within the brain that reveal its organization.
Credit: Duke Center for In Vivo Microscopy
DURHAM, N.C. – Magnetic resonance imaging (MRI) is how we visualize soft, watery tissue that is hard to image with X-rays. But while an MRI provides good enough resolution to spot a brain tumor, it needs to be a lot sharper to visualize microscopic details within the brain that reveal its organization.
In a decades-long technical tour de force lead by Duke’s Center for In Vivo Microscopy with colleagues at the University of Tennessee Health Science Center, University of Pennsylvania, University of Pittsburgh and Indiana University, researchers took up the gauntlet and improved the resolution of MRI leading to the sharpest images ever captured of a mouse brain.
Coinciding with the 50th anniversary of the first MRI, the researchers generated scans of a mouse brain that are dramatically crisper than a typical clinical MRI for humans, the scientific equivalent of going from a pixelated 8-bit graphic to the hyper-realistic detail of a Chuck Close painting.
A single voxel of the new images – think of it as a cubic pixel – measures just 5 microns. That’s 64 million times smaller than a clinical MRI voxel.
Although the researchers focused their magnets on mice instead of humans, the refined MRI provides an important new way to visualize the connectivity of the entire brain at record-breaking resolution. The researchers say new insights from mouse imaging will in turn lead to a better understanding of conditions in humans, such as how the brain changes with age, diet, or even with neurodegenerative diseases like Alzheimer’s.
“It is something that is truly enabling. We can start looking at neurodegenerative diseases in an entirely different way,” said G. Allan Johnson, Ph.D., the lead author of the new paper and the Charles E. Putman University Distinguished professor of radiology, physics and biomedical engineering at Duke.
Johnson’s excitement is a long time coming. The team’s new work, appearing April 17 in the Proceedings of the National Academy of Sciences, is the culmination of nearly 40 years of research at the Duke Center for In Vivo Microscopy.
Over the four decades, Johnson, his engineering graduate students and his many collaborators at Duke and afar refined many elements that, when all combined, made the revolutionary MRI resolution possible.
Some of the key ingredients include an incredibly powerful magnet (most clinical MRIs rely on a 1.5 to 3 Tesla magnet; Johnson’s team uses a 9.4 Tesla magnet), a special set of gradient coils that are 100 times stronger than those in a clinical MRI and help generate the brain image, and a high-performance computer equivalent to nearly 800 laptops all cranking away to image one brain.
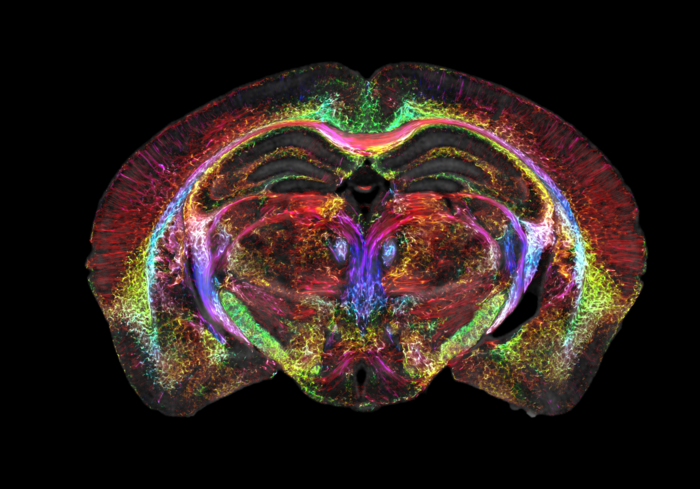
After Johnson and his team “scan the daylights out of it,” they send off the tissue to be imaged using a different technique called light sheet microscopy. This complementary technique gives them the ability to label specific groups of cells across the brain, such as dopamine-issuing cells to watch the progression of Parkinson’s disease.
The team then maps the light sheet pictures, which give a highly accurate look at brain cells, onto the original MRI scan, which is much more anatomically accurate and provides a vivid view of cells and circuits throughout the entire brain.
With this combined whole brain data imagery, researchers can now peer into the microscopic mysteries of the brain in ways never possible before.
One set of MRI images shows how brain-wide connectivity changes as mice age, as well as how specific regions, like the memory-involved subiculum, change more than the rest of the mouse’s brain.
Another set of images showcases a spool of rainbow-colored brain connections that highlight the remarkable deterioration of neural networks in a mouse model of Alzheimer’s disease.
The hope is that by making the MRI an even higher-powered microscope, Johnson and others can better understand mouse models of human diseases, such as Huntington’s disease, Alzheimer’s, and others. And that should lead to a better understanding of how similar things function or go awry in people.
“Research supported by the National Institute of Aging uncovered that modest dietary and drug interventions can lead to animals living 25% longer,” Johnson said. “So, the question is, is their brain still intact during this extended lifespan? Could they still do crossword puzzles? Are they going to be able to do Sudoku even though they’re living 25% longer? And we have the capacity now to look at it. And as we do so, we can translate that directly into the human condition.”
This research was supported by the National Institutes of Health (R01-AG070913391, R01-NS096729, P41EB015897, S10OD010683).
CITATION: “Merged Magnetic Resonance And Light Sheet Microscopy Of The Whole Mouse Brain,” G. Allan Johnson, Yuqi Tian, David G. Ashbrook, Gary P. Cofer, James J. Cook, James C. Gee, Adam Hall, Kathryn Hornburg, Yi Qi, Fang-Cheng Yeh, Nian Wang, Leonard E. White, Robert W. Williams. Proceedings of the National Academy of Sciences, April 17, 2023. DOI: 10.1073/pnas.2218617120
Journal
Proceedings of the National Academy of Sciences
DOI
10.1073/pnas.2218617120
Method of Research
Imaging analysis
Subject of Research
Animals
Article Title
Merged Magnetic Resonance And Light Sheet Microscopy Of The Whole Mouse Brain
Article Publication Date
17-Apr-2023



