Competition between nerve fibers for space in rodent brains shapes complex circuits called ‘whisker barrels’, providing new clues about the role of genes in brain development
Credit: James et al. (CC BY 4.0)
Complex brain circuits in rodents can organise themselves with genetics playing only a secondary role, according to a new computer modelling study published today in eLife.
The findings help answer a key question about how the brain wires itself during development. They suggest that simple interactions between nerve cells contribute to the development of complex brain circuits, so that a precise genetic blueprint for brain circuitry is unnecessary. This discovery may help scientists better understand disorders that affect brain development and inform new ways to treat conditions that disrupt brain circuits.
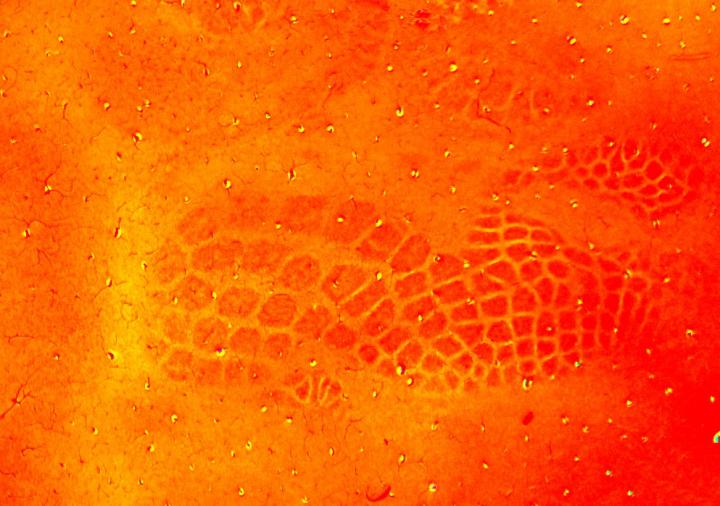
The circuits that help rodents process sensory information collected by their whiskers are a great example of the complexity of brain wiring. These circuits are organised into cylindrical clusters or ‘whisker barrels’ that closely match the pattern of whiskers on the animal’s face.
“The brain cells within one whisker barrel become active when its corresponding whisker is touched,” explains lead author Sebastian James, Research Associate at the Department of Psychology, University of Sheffield, UK. “This precise mapping between the individual whisker and its brain representation makes the whisker-barrel system ideal for studying brain wiring.”
James and his colleagues used computer modelling to determine if this pattern of brain wiring could emerge without a precise genetic blueprint. Their simulations showed that, in the cramped quarters of the developing rodent brain, strong competition for space between nerve fibers originating from different whiskers can cause them to concentrate into whisker-specific clusters. The arrangement of these clusters to form a map of the whiskers is assisted by simple patterns of gene expression in the brain tissue.
The team also tested their model by seeing if it could recreate the results of experiments that track the effects of a rat losing a whisker on its brain development. “Our simulations demonstrated that the model can be used to accurately test how factors inside and outside of the brain can contribute to the development of cortical fields,” says co-author Leah Krubitzer, Professor of Psychology at the University of California, Davis, US.
The authors suggest that this and similar computational models could be adapted to study the development of larger, more complex brains, including those of humans.
“Many of the basic mechanisms of development in the rodent barrel cortex are thought to translate to development in the rest of cortex, and may help inform research into various neurodevelopmental disorders and recovery from brain injuries,” concludes senior author Stuart Wilson, Lecturer in Cognitive Neuroscience at the University of Sheffield. “As well as reducing the number of animal experiments needed to understand cortical development, exploring the parameters of computational models like ours can offer new insights into how development and evolution interact to shape the brains of mammals, including ourselves.”
###
Reference
The paper ‘Modeling the emergence of whisker barrels’ can be freely accessed online at https:/
Media contact
Emily Packer, Media Relations Manager
eLife
[email protected]
01223 855373
About eLife
eLife is a non-profit organisation created by funders and led by researchers. Our mission is to accelerate discovery by operating a platform for research communication that encourages and recognises the most responsible behaviours. We work across three major areas: publishing, technology and research culture. We aim to publish work of the highest standards and importance in all areas of biology and medicine, including Computational and Systems Biology and Developmental Biology, while exploring creative new ways to improve how research is assessed and published. We also invest in open-source technology innovation to modernise the infrastructure for science publishing and improve online tools for sharing, using and interacting with new results. eLife receives financial support and strategic guidance from the Howard Hughes Medical Institute, the Knut and Alice Wallenberg Foundation, the Max Planck Society and Wellcome. Learn more at https:/
To read the latest Computational and Systems Biology research published in eLife, visit https:/
And for the latest in Developmental Biology, see https:/
Media Contact
Emily Packer
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




