Herpes simplex virus type 1 (HSV-1) is notorious not only for causing cold sores but also for its potential to induce herpes simplex encephalitis (HSE), a rare yet often fatal inflammation of the brain. Despite the severity of HSE, therapeutic options remain disappointingly scarce, primarily due to the virus’s ability to elude the host immune system within the central nervous system. A pioneering study led by Professor Yasushi Kawaguchi and his team at The Institute of Medical Science, The University of Tokyo, has shed light on the complex molecular interplay that enables HSV-1 to evade intrinsic antiviral defenses in the brain. Their findings, soon to be published in Nature Microbiology, unravel a novel mechanism of viral immune escape and present a promising strategy to restore innate immunity and combat this devastating neurological infection.
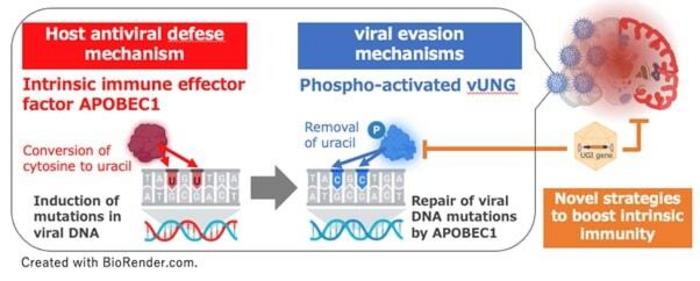
At the heart of this viral subterfuge lies a critical interaction between the host’s antiviral protein APOBEC1 (apolipoprotein B mRNA editing enzyme, catalytic polypeptide-1) and a viral enzyme encoded by HSV-1 known as uracil-DNA glycosylase (vUNG). APOBEC1 belongs to a family of cytidine deaminases capable of inducing mutagenic lesions in viral DNA, thereby crippling viral replication by introducing lethal mutations. Ordinarily, this antiviral editing serves as a formidable barrier to infection; however, HSV-1 has evolved the vUNG enzyme to counteract APOBEC1’s mutagenic activity, thereby preserving viral genome integrity and facilitating viral proliferation in the brain.
Professor Kawaguchi’s team embarked on a detailed molecular investigation which revealed that vUNG enzymatically excises uracil bases that arise from APOBEC1-mediated deamination in the viral genome. This excision prevents the accumulation of mutations that would otherwise compromise viral replication. Through ingenious experimental design utilizing genetically modified viral strains, the researchers demonstrated that the biological activity of vUNG is modulated by phosphorylation at serine residue 302. Mutant HSV-1 which harbors a non-phosphorylatable amino acid substitution at this site exhibits impaired vUNG activity and consequently fails to evade APOBEC1-mediated mutation. This impairment correlates with diminished viral load in infected murine brains and significantly improved survival rates.
.adsslot_pADrt1IOZh{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_pADrt1IOZh{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_pADrt1IOZh{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Building upon this mechanistic insight, the researchers devised a gene therapy approach to artificially inhibit vUNG in vivo. Using an adeno-associated virus (AAV) delivery system, they introduced a viral glycosylase inhibitor protein (UGI) specifically designed to bind and neutralize vUNG activity. Pre-treatment of mice with the AAV-UGI vector prior to HSV-1 exposure restored APOBEC1’s antiviral function, dramatically reducing viral replication in the brain and conferring robust protection against fatal encephalitis. This strategy marks a paradigm shift away from traditional antiviral therapies that target viral replication directly—it leverages the reactivation of intrinsic host immunity by thwarting viral immune evasion tactics.
Crucially, the protective effect of AAV-UGI was dependent on the presence of APOBEC1. When mice genetically deficient in APOBEC1 received the same AAV-UGI treatment, viral infection remained uncontrolled and lethal. This finding underscores the indispensable role of APOBEC1 in mediating the observed antiviral immunity and suggests that therapeutic success hinges on the interplay between host deaminases and viral DNA repair pathways. It also validates a novel class of antivirals that do not target the virus per se, but modulate the host-virus interface for therapeutic gain.
This study’s implications extend beyond HSV-1 encephalitis. Many neurotropic viruses have evolved sophisticated methods to circumvent host intrinsic immunity, often targeting DNA or RNA editing enzymes. By elucidating the role of vUNG phosphorylation in activating its immune-suppressing function, the research provides a molecular target for drug development. Inhibitors or gene therapies analogous to AAV-UGI could be tailored to diverse neurotropic viruses which employ similar glycosylase-mediated immune suppression, thereby broadening the impact of this work.
Moreover, reactivating intrinsic immunity via inhibition of viral countermeasures has potential clinical advantages. Unlike high-dose antiviral drugs, which often cause adverse effects and risk promoting resistant viral variants, this therapeutic strategy capitalizes on naturally evolved cellular mechanisms. As a result, it may offer a safer, more sustainable approach to managing viral encephalitis, especially in patients unresponsive to current treatments. This host-focused paradigm could revolutionize how we address infectious diseases within the central nervous system, where immune privilege complicates treatment.
From a translational standpoint, the use of AAV vectors as gene delivery vehicles aligns well with advances in gene therapy, where safe, targeted expression of therapeutic proteins in the brain is becoming increasingly feasible. The demonstration that AAV-mediated delivery of UGI effectively suppresses viral infection in mice paves the way for preclinical development and future clinical trials. Safety, dosing, and long-term immunological effects will require meticulous evaluation, but this approach represents a compelling new frontier in antiviral therapy.
The revelation that phosphorylation at a single serine residue is pivotal for vUNG’s immunosuppressive activity adds a layer of specificity that could inform precision drug design. Small molecules or biologics inhibiting the kinase responsible for this post-translational modification might serve as adjunctive therapies. Furthermore, delineating the cellular signaling pathways leading to vUNG phosphorylation may yield novel targets to disrupt viral immune evasion.
In conclusion, this landmark study exposes the intricate molecular dialogue between HSV-1 and its host within the brain, highlighting how viral uracil-DNA glycosylase undermines APOBEC1-mediated immunity. The innovative gene therapy approach developed to restore intrinsic antiviral defenses heralds a promising avenue for treating herpes simplex encephalitis. As we grapple with the challenges of neurotropic viral infections, harnessing and reactivating the body’s own antiviral repertoire offers a beacon of hope for more effective, durable, and less toxic treatments.
Subject of Research: Animals
Article Title: Herpes simplex virus-1 evades APOBEC1-mediated immunity via its uracil DNA glycosylase in mice
News Publication Date: June 3, 2025
Web References:
https://www.doi.org/10.1038/s41564-025-02026-3
References:
Kato, A., Harima, H., Tsunekawa, Y., et al. Herpes simplex virus-1 evades APOBEC1-mediated immunity via its uracil DNA glycosylase in mice. Nature Microbiology (2025). DOI: 10.1038/s41564-025-02026-3
Image Credits:
Professor Yasushi Kawaguchi, The University of Tokyo, Japan
Keywords:
Virology, Immunology, Microbiology, Molecular biology, Genetics, Biotechnology, Infectious diseases, Medical treatments, Pharmacology, Encephalitis, Cell biology
Tags: APOBEC1 antiviral protein functionsboosting brain immunity against infectionsbrain inflammation from herpesherpes simplex virus treatment researchHSV-1 immune evasion mechanismsinnate immunity restoration techniquesNature Microbiology HSV-1 study findingsneurological impacts of HSV-1the interplay of host and viral proteinstherapeutic strategies for herpes simplex encephalitisuracil-DNA glycosylase role in HSV-1viral enzyme targets for therapy