In a groundbreaking advancement addressing one of the most formidable challenges in oncology, recent research has unveiled a novel therapeutic strategy capable of surmounting resistance to immunotherapy in melanoma. Melanoma, an aggressive form of skin cancer, often develops resistance to immune checkpoint inhibitors such as PD-1 blockade, leaving patients with limited treatment options. The new study elucidates how inhibiting the RAS/MEK/PI3K signaling pathways amplifies the efficacy of CD40 agonists by precisely targeting a suppressive B cell subset known as CD11b+ regulatory B cells (Bregs). This dual approach not only augments anti-tumor immunity but also offers a promising avenue to counteract PD-1 resistance, a pressing issue in current cancer therapeutics.
The interplay between tumor cells and the immune microenvironment plays a critical role in cancer progression and response to treatment. Bregs, particularly the subset expressing CD11b, have emerged as significant modulators within the tumor milieu, capable of dampening immune responses and facilitating tumor evasion from immunosurveillance. Previous attempts to harness the immune system against melanoma have largely focused on T cell activation, often overlooking the suppressive impact of Bregs. The latest findings highlight that these CD11b+ Bregs are instrumental in fostering an immunosuppressive niche, thereby limiting the effectiveness of PD-1 blockade therapies.
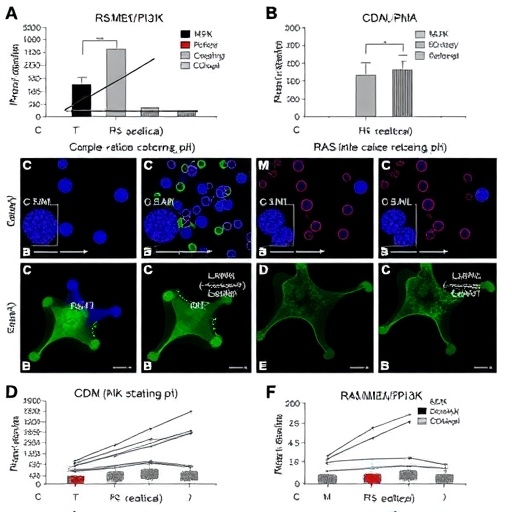
At the molecular level, the RAS/MEK/PI3K signaling axis is a well-established regulator of various cellular processes, including proliferation, survival, and immune modulation. Hyperactivation of this pathway not only drives melanoma progression but also appears to sustain the suppressive function of CD11b+ Bregs. By pharmacologically inhibiting components of this pathway, researchers observed a significant reduction in the immunosuppressive capacity of these Bregs. This, in turn, allowed for a more potent activation of anti-tumor immune mechanisms when combined with CD40 agonism.
CD40 is a co-stimulatory protein found on antigen-presenting cells, including B cells, dendritic cells, and macrophages. Agonists targeting CD40 have shown promise in enhancing immune responses against tumors by promoting T cell priming and activation. Yet, their efficacy has been limited by the presence of regulatory immune cells that curb overall immune activation. The study reveals that combining CD40 stimulation with RAS/MEK/PI3K pathway inhibitors effectively dismantles the suppressive shield imposed by CD11b+ Bregs, unleashing a robust and sustained anti-tumor response.
Using melanoma models resistant to PD-1 blockade, the researchers demonstrated that this combination therapy led to pronounced tumor regression and prolonged survival. Importantly, this therapeutic synergy was not merely additive but synergistic, underscoring the potential of targeting both intrinsic tumor signaling and its extrinsic immunosuppressive mechanisms. Molecular analyses confirmed the downregulation of immunosuppressive markers and a concurrent increase in effector T cell infiltration within the tumor microenvironment.
This study also sheds light on the heterogeneity within B regulatory cells and the necessity of targeting specific subsets to achieve effective immunomodulation. Previous broad-spectrum B cell depletion strategies risked compromising beneficial humoral immunity; however, the selective targeting of CD11b+ Bregs via pathway inhibition circumvents this issue, maintaining overall immune competence while alleviating suppression. The precision of this approach may pave the way for more tailored immunotherapies with fewer adverse effects.
Furthermore, the translational implications of these findings are profound. Patients with melanoma who fail to respond to PD-1 inhibitors currently face poor prognoses and limited therapeutic alternatives. The dual intervention targeting RAS/MEK/PI3K and activating CD40 represents a potential breakthrough, offering a mechanism to overcome resistance and restore immune-mediated tumor control. Clinical trials investigating this combinatorial strategy could redefine standards of care in melanoma and possibly other malignancies exhibiting similar immunosuppressive pathways.
The mechanistic insights provided by this research also encourage a reassessment of combination immunotherapy design. While checkpoint blockade revolutionized cancer treatment, the contribution of other immune cells such as Bregs has been underappreciated. Integrating the modulation of these cells may optimize response rates and durability across diverse tumor types. The specific inhibition of signaling pathways like RAS/MEK/PI3K could emerge as a cornerstone in next-generation immunotherapies.
Moreover, the study highlights the importance of dissecting tumor-immune cell interactions to identify novel checkpoints beyond PD-1 and CTLA-4. It becomes evident that intricate signaling crosstalk within the tumor microenvironment profoundly influences therapeutic outcomes. Targeting signaling cascades in immune regulatory cells alongside activating stimulatory receptors holds tremendous promise for reinvigorating anti-cancer immunity.
Future research directions inspired by these findings include investigating optimal dosing regimens, potential biomarkers for patient stratification, and the exploration of combinatorial therapies incorporating other immune modulators or targeted agents. The ability to precisely manipulate immune subsets while minimizing systemic toxicity will be critical to the successful clinical translation of this approach.
In conclusion, this pioneering study offers a compelling strategy to counteract melanoma resistance to PD-1 blockade by combining RAS/MEK/PI3K pathway inhibitors with CD40 agonists, selectively targeting suppressive CD11b+ Bregs. This multifaceted approach reinvigorates anti-tumor immunity, facilitates robust T cell responses, and leads to significant tumor control in preclinical models. As melanoma continues to pose significant clinical challenges, such innovative therapies herald a new era of precision immuno-oncology, promising improved outcomes for patients with resistant tumors.
With an eye toward the future, integrating pathway inhibition and immune activation strategies underscores the evolving complexity and sophistication of cancer immunotherapy. As researchers delve deeper into the tumor microenvironment’s nuances, therapies that intelligently exploit these insights will transform the landscape of cancer treatment. This landmark discovery serves as a beacon of hope, illuminating pathways to surmount immune resistance and unlock the full potential of the immune system against cancer.
By harnessing a focused attack on regulatory B cells combined with immune-stimulating agonists, this research not only expands the therapeutic arsenal against melanoma but also charts a course toward overcoming resistance mechanisms pervasive across malignancies. The convergence of molecular targeting and immunotherapy exemplifies the next frontier in oncology poised to deliver durable, long-lasting remissions and, ultimately, cures.
Subject of Research: Melanoma immunotherapy resistance and approaches to overcome PD-1 blockade resistance through targeting CD11b+ regulatory B cells using RAS/MEK/PI3K pathway inhibition combined with CD40 agonism.
Article Title: RAS/MEK/PI3K pathway inhibition augments response to CD40 agonism by targeting CD11b+ Bregs thereby overcoming melanoma PD1-resistance.
Article References:
Yan, C., Luo, W., Yang, J. et al. RAS/MEK/PI3K pathway inhibition augments response to CD40 agonism by targeting CD11b+ Bregs thereby overcoming melanoma PD1-resistance. Nat Commun 17, 162 (2026). https://doi.org/10.1038/s41467-025-67315-1
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41467-025-67315-1
Tags: anti-tumor immunity enhancementcancer treatment advancementsCD11b regulatory B cellsCD40 agonist therapyimmune checkpoint inhibitorsimmunosuppressive B cell subsetsmelanoma immunotherapy resistancemelanoma treatment strategiesovercoming cancer therapy resistancePD-1 blockade limitationsRAS MEK PI3K signaling pathwaystumor immune microenvironment