The work of a researcher involves not only continuous pursuit of new discoveries but also careful attention to conventional, established knowledge. Sometimes, reliable and seemingly proven methods have characteristics that were previously overlooked.
The work of a researcher involves not only continuous pursuit of new discoveries but also careful attention to conventional, established knowledge. Sometimes, reliable and seemingly proven methods have characteristics that were previously overlooked.
A group of researchers led by Alexander Tonevitsky, Dean of the Faculty of Biology and Biotechnology at HSE University, have discovered such characteristics in the microRNA overexpression method. The scientists found that, in some cases, the results of experiments using this method may be incorrect, and these errors are very difficult to detect.
MicroRNAs (miRNAs) are small RNA molecules, approximately 20 to 25 nucleotides in length. They play an important role in regulating gene expression by determining how much protein will be synthesised in a cell from a specific molecule of messenger RNA (mRNA). This regulation is facilitated by a short fragment within the miRNA, which can bind to the target mRNA molecule if it finds a reverse complement (biologically compatible) sequence. When this occurs, protein synthesis from the mRNA is halted, leading to a decrease in gene expression.
As diseases, including cancers, progress, changes in microRNA expression levels are observed. Specifically, in prostate cancer, the number of miR-93-5p microRNAs increases, and higher levels of their expression are associated with greater aggressiveness of the disease. By significantly increasing the number of miRNA in laboratory cells, researchers can gain a clearer understanding of the processes associated with elevated expression of this miRNA.
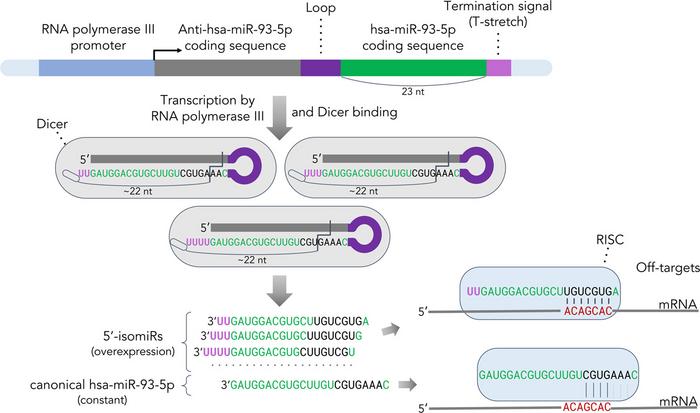
A common approach for miRNA overexpression involves initially increasing the amount of precursor RNA in cells—a longer molecule from which miRNA is subsequently generated through cleavage by the Dicer enzyme. This is a natural process for the cell. However, the sequence of miRNAs formed as a result depends on how accurately the Dicer enzyme cleaves the precursor molecule.
The authors of the paper studied how the Dicer enzyme cleaves the molecule. They encoded the desired sequence in the precursor molecule, anticipating that Dicer would accurately cleave it as intended. However, it turned out that Dicer does not always operate as scientists expect. The enzyme primarily functions like a molecular ruler, consistently measuring a length of 22 nucleotides. During the synthesis of the precursor molecule, one or more uracils (a nucleotide base unique to RNA) are typically added to the end of the sequence. As a result, if a given miRNA sequence is longer than 19 nucleotides, the added uracils cause Dicer to cleave the molecule at an unintended location. This shift leads to the formation of miRNA isoforms.
To investigate the cause of the shift in cleavage position of the precursor molecule, the scientists experimentally set several miRNA sequences of varying lengths, including a 23-nucleotide chain corresponding to the miR-93-5p miRNA. Using sequencing—a method that fully deciphers the nucleotide sequence of RNA or DNA molecules—the scientists observed that the added uracils in chains longer than 19 nucleotides cause a shift in the cleavage position.
The formation of miR-93-5p miRNA isoforms resulted in a decrease in the expression of the HMGA1 gene, which plays a role in disrupting genetic information transmission during cell division and in regulating gene expression. However, HMGA1 was not the target of the standard form of miR-93-5p. Without knowledge of miRNA isoform formation, one might draw incorrect conclusions about the molecular mechanisms of the studied miRNA in prostate cancer.
‘Isoforms may target the wrong mRNA molecules instead of those intended in the experiment, leading to the suppression of unintended genes. Understanding this peculiarity is crucial for both basic research and medical applications,’ according to Diana Maltseva, Head of the International Laboratory of Microphysiological Systems at HSE University.
Scientists worldwide use the miRNA overexpression method in their experiments. Typically, the accuracy of the results is verified using polymerase chain reaction (PCR). However, this method was not sufficiently sensitive in this case.
‘Our study has shown that only sequencing can reveal the shift in the cleavage position. Unfortunately, sequencing is a relatively expensive method, and not all laboratories can afford it. Therefore, it is crucial to develop new methods for miRNA overexpression and to design experiments carefully,’ Maltseva comments.
Journal
Biochimica et Biophysica Acta
DOI
10.1016/j.bbagrm.2024.195046
Method of Research
Experimental study
Subject of Research
Cells
Article Title
References (66) Elsevier Biochimica et Biophysica Acta (BBA) – Gene Regulatory Mechanisms Volume 1867, Issue 3 , September 2024, 195046 Biochimica et Biophysica Acta (BBA) – Gene Regulatory Mechanisms Incautious design of shRNAs for stable overexpression of miRNAs could result in generation of undesired isomiRs
Article Publication Date
3-Sep-2024