Biochemists discover highly selective phage activation based on signal molecule
This targeted control of phages provides entirely new biotechnological and therapeutic approaches, e.g. for phage therapies. The results produced in the context of an ERC grant have been published in the Journal of the American Chemical Society.
Warfare between microbes
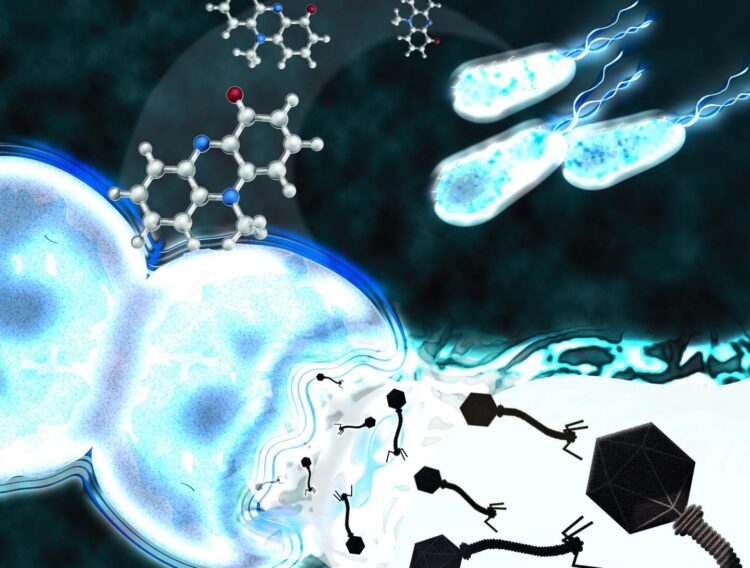
“We already know that phages decisively influence the population dynamics of bacteria and that microorganisms compete by using chemical weapons,” says Thomas Böttcher, Professor of Microbial Biochemistry of the Faculty of Chemistry and the Centre of Microbiology and Environmental Systems Science. “We now wanted to investigate whether, in the complex microbial ecosystems, there are also microbes that specifically activate phages in order to use them against their competitors.”
Indeed, the researchers could show that the bacterium Pseudomonas aeruginosa produces large amounts of a signal molecule that triggers the conversion of a phage, residing in a strain of the species Staphylococcus aureus, from a quiet companion into a deadly parasite.
Highly selective phage activation
“We were completely surprised to find that the chemical compound pyocyanin, which we were able to isolate and synthesise, only specifically activated one of several phages of Staphylococcus aureus. Pyocyanin is therefore a highly selective agent,” says co-author Magdalena Jancheva.
The drug mitomycin C induces DNA damage in bacterial cells and causes phages to leave their dying host, but according to Thomas Böttcher, “it activates all phages in the bacteria in a non-selective manner”. The researchers also observed that pyocyanin releases even more phages in Staphylococcus aureus than mitomycin C, pyocyanin therefore had an “remarkably strong effect”.
Discovery provides new perspectives
The bacterial species Pseudomonas aeruginosa and Staphylococcus aureus occupy the same ecological niche in the human body. As pathogens, they frequently occur in the lungs of patients with cystic fibrosis, a congenital metabolic disease. Staphylococcus bacteria dominate at a young age, while Pseudomonas bacteria become more prevalent with increasing age.
The current study demonstrates the efficiency of activation of latent phages by chemical signaling agents in the battle for space and resources between bacterial strains. It provides the first evidence that chemical signaling agents can exhibit selectivity for specific phages in a polylysogenic bacterial strain. Here, the activated phage (phiMBL3) revealed a previously unknown molecular switch through which the signaling agent acts.
###
Publication in “JACS – Journal of the American Chemical Society“:
A Metabolite of Pseudomonas Triggers Prophage-Selective Lysogenic to Lytic Conversion in Staphylococcus aureus. Magdalena Jancheva & Thomas Böttcher, in: Journal of the American Chemical Society 2021, DOI: https:/
Media Contact
Dr. Thomas Böttcher
[email protected]
Related Journal Article
http://dx.