A pioneering breakthrough from Toho University promises to revolutionize the identification of bioactive compounds capable of combating the deleterious effects of advanced glycation end products (AGEs). A team of scientists has developed a highly sophisticated automated program designed to accelerate the discovery of natural compounds that exhibit anti-glycation properties — a critical step forward in battling age-associated chronic diseases. The findings, published on May 3, 2025, in the prestigious journal Analytical Chemistry, detail a new Carbonyl-Trapping Mechanism-Based Automatic Mining (CTM-AM) strategy that harnesses advanced analytical technologies integrated with computational power.
Glycation is a biochemical process where sugar molecules, such as methylglyoxal (MGO), react non-enzymatically with proteins, lipids, and nucleic acids, leading to the formation of AGEs. These compounds contribute to the pathogenesis of diabetes, neurodegeneration, cardiovascular disorders, and other chronic ailments. Consequently, the search for natural products possessing potent anti-glycation effects is of paramount clinical importance. However, traditional methods for screening such compounds from complex natural matrices have been labor-intensive, time-consuming, and often lacked precision. The newly developed CTM-AM strategy addresses these limitations by integrating a carbonyl-trapping mechanism with cutting-edge high-performance liquid chromatography coupled to mass spectrometry (LC-MS).
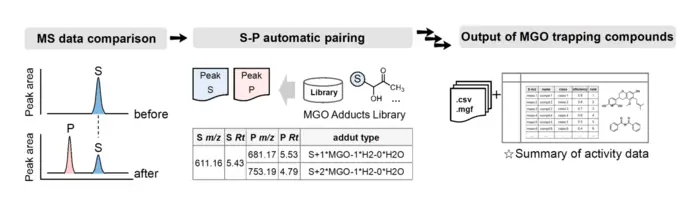
The heart of this innovative approach is an automated computational program uniquely tailored to analyze LC-MS data obtained from medicinal plant extracts. Utilizing sophisticated algorithms, the program identifies and characterizes 171 methylglyoxal-trapping compounds with high confidence. This rapid and reliable identification process represents a significant leap over conventional manual data analysis techniques. By automating this process, researchers can now rapidly mine complex datasets to pinpoint targeted bioactive molecules that interrupt glycation pathways, thus expediting drug discovery pipelines.
.adsslot_4AYPpBi6zI{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_4AYPpBi6zI{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_4AYPpBi6zI{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Technically, the CTM-AM strategy capitalizes on the reactivity between carbonyl groups of reactive dicarbonyl species like MGO and nucleophilic compounds present in natural products. This trapping mechanism forms stable adducts, which serve as molecular fingerprints identifiable by mass spectrometry. The program then automatically mines the generated mass spectral data, discerning subtle spectral features indicative of these adducts. This innovative fusion of experimental and computational techniques overcomes previous analytical challenges posed by the structural diversity and complexity of natural compounds, ensuring both specificity and sensitivity in compound detection.
This fresh methodological framework also exemplifies the increasing role of artificial intelligence and machine learning algorithms in chemical biology. The automated program’s capacity to learn and adapt to datasets improves its efficiency and accuracy in recognizing patterns consistent with potent anti-glycation molecules. Moreover, this technology substantially reduces human bias and error, enhancing reproducibility of results—a critical concern in natural product research where sample complexity can obscure subtle bioactive constituents.
Beyond pure analytical innovation, the CTM-AM methodology holds immense translational value. Identifying natural products with high affinity to trap MGO could lead to the development of novel therapeutics aimed at mitigating the progression of diabetic complications, Alzheimer’s disease, and other AGE-related conditions. Previously, therapeutic options targeting AGEs were limited, partly due to the difficulty in pinpointing effective compounds. With this new automated mining platform, pharmaceutical and nutraceutical industries can expedite the screening and validation phases, accelerating the journey from natural extract to clinically relevant drug.
The team from Toho University has validated the efficacy of their program using diverse medicinal plant extracts. This real-world application underscores the program’s versatility in coping with the vast chemical heterogeneity encountered in botanicals, which historically posed significant challenges for conventional screening methods. The ability to navigate complex LC-MS datasets with precision highlights the robustness of the automated system, which integrates seamlessly into existing analytical workflows without requiring extensive manual intervention.
One of the critical strengths of this approach lies in its combination of mechanistic chemical insight with advanced computational design. While the carbonyl-trapping chemistry is well-documented, embedding it within an automated mining platform elevates its utility to an unprecedented scale. This synergy exemplifies modern interdisciplinary research—where chemical theory informs computational tool development, resulting in practical solutions primed for high-throughput natural product discovery.
Furthermore, the implications of this research extend to broader natural product science and metabolomics fields. Automated data mining platforms such as CTM-AM can be adapted to target different biochemical mechanisms beyond carbonyl trapping, facilitating the exploration of diverse bioactivities within natural product libraries. This modular adaptability opens avenues for discovering novel classes of functional compounds vital for health and disease management, emphasizing the flexibility of this technology.
The study was led by Wenjun Qi, Mi Zhang, Takashi Kikuchi, Kouharu Otsuki, and Wei Li, representing a multidisciplinary effort blending expertise in analytical chemistry, computational biology, and pharmacognosy. Their collaborative work not only sets a new benchmark for rapid screening of anti-glycation agents but also exemplifies the importance of cross-field partnerships in accelerating innovation. The published work incorporates comprehensive supporting data, transparency in methodology, and an open declaration of no conflicts of interest, ensuring its credibility within the scientific community.
Looking ahead, the research team envisions that this platform could be further refined by incorporating deep learning frameworks to enhance predictive capabilities and expand compound identification beyond methylglyoxal-trapping agents. Such advancement could revolutionize natural resource utilization, empowering researchers globally to tap into the vast, largely uncharted chemical diversity present in nature’s pharmacopeia. The democratization of such tools promises to catalyze discovery across ecology, medicine, and biotechnology sectors.
In an era where chronic metabolic diseases continue to rise, innovations like the CTM-AM automated mining strategy provide hope for more effective preventative and therapeutic measures. By enabling rapid and confident identification of anti-glycation compounds, this work paves the way for scalable advancements in natural product-based health interventions. The successful synthesis of cutting-edge analytical chemistry with computational prowess demonstrated in this study marks a defining moment for bioactive compound exploration.
This discovery also reinforces the critical role of methodological innovation in natural product research, traditionally hampered by laborious experimental setups and ambiguous results. As the global health community seeks novel solutions to combat aging-related pathologies, tools like CTM-AM that streamline and enhance discovery processes will be indispensable. The future of anti-glycation research, integrating automated detection with mechanistic chemistry, promises to deliver therapeutics that improve quality of life for millions worldwide.
The publication of this research in Analytical Chemistry solidifies its scientific importance and provides open access to methodologies that researchers can adopt and adapt. This transparency fuels further scientific dialogue and development in the field, potentially leading to collaborative enhancements and broader application of automated mining technologies across a spectrum of biomedical challenges.
Subject of Research: Not applicable
Article Title: A Carbonyl-Trapping Mechanism-Based Automatic Mining (CTM-AM) Strategy for Accelerating the Discovery of Natural Products with Anti-Advanced Glycation End Products Activity
News Publication Date: May 3, 2025
Web References: http://dx.doi.org/10.1021/acs.analchem.5c00216
References:
Qi, W., Zhang, M., Kikuchi, T., Otsuki, K., & Li, W. (2025). A Carbonyl-Trapping Mechanism-Based Automatic Mining (CTM-AM) Strategy for Accelerating the Discovery of Natural Products with Anti-Advanced Glycation End Products Activity. Analytical Chemistry, 97(18), 9836–9847. https://doi.org/10.1021/acs.analchem.5c00216
Image Credits: Wei Li
Keywords: Anti-glycation compounds, advanced glycation end products, automated data mining, methylglyoxal trapping, natural product discovery, high-performance liquid chromatography, mass spectrometry, carbonyl-trapping mechanism, CTM-AM, analytical chemistry, computational chemistry, medicinal plant extracts
Tags: advanced glycation end productsanalytical chemistry innovationsanti-glycation compoundsautomated identification of bioactive substancesCarbonyl-Trapping Mechanism-Based Automatic Miningchronic disease prevention strategiescomputational methods in drug discoverydiabetes and neurodegeneration researchglycation process and implicationshigh-performance liquid chromatography techniquesnatural sources of anti-glycation agentsToho University scientific breakthroughs