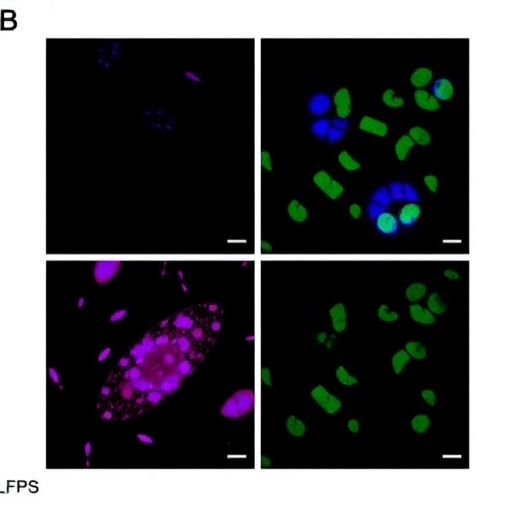
In a groundbreaking study poised to reshape our understanding of tumor biology and microbiome interactions, researchers have unearthed the intricate ways in which activation of the transcription factor ATF6 alters lipid metabolism in the colon, sparking an adaptive response in tumor-associated microbial communities. This discovery not only illuminates a hitherto obscured metabolic axis within the tumor microenvironment but also evokes new avenues for therapeutic interventions targeting both cancer cells and their symbiotic microbes.
ATF6, well known for its central role in the unfolded protein response (UPR) during endoplasmic reticulum stress, has traditionally been studied in the context of cellular homeostasis and survival mechanisms under conditions of proteotoxic stress. However, the novel insights presented here extend ATF6’s significance far beyond its classical functions. The study demonstrates how ATF6 activation orchestrates a profound reprogramming of lipid metabolic pathways within colonic epithelial cells—and crucially, how these lipid alterations serve as biochemical cues for resident microbial populations within evolving tumors to adapt and thrive.
The metabolic plasticity of tumor cells is a hallmark of cancer progression, often involving rewiring of carbohydrate and lipid metabolism to support rapid proliferation and survival under hostile conditions. This research specifically addresses the lipid-centric metabolic changes induced by ATF6 signaling. By employing state-of-the-art lipidomics, metabolomics, and single-cell transcriptomics, the investigators characterized a signature metabolic profile distinguished by shifts in fatty acid synthesis, elongation, and desaturation pathways. These shifts culminate in an altered landscape of colonic lipids that reshape the niche for nearby microbial communities.
Perhaps most strikingly, the study reveals that tumor-associated microbes do not passively endure these metabolic changes but actively remodel their own metabolic functions in response to the tumor-induced lipid milieu. This adaptive microbial behavior is demonstrated through metagenomic sequencing and functional assays, which show specific microbial taxa expanding their capacity for lipid utilization and remodeling their membrane composition to coexist within this modified environment. Such microbial plasticity hints at a dynamic metabolic dialogue between host tumor cells and their microbial counterparts with significant implications for tumor progression and response to therapy.
The consequences of this metabolic crosstalk reach beyond mere coexistence. Altered microbial communities can, in turn, influence tumor biology by modulating local immune responses, producing bioactive metabolites, and affecting the bioavailability of lipids and other nutrients. This feedback loop, initiated by ATF6-driven lipid changes in colonic tumors, underscores the complexity of the tumor ecosystem and elevates the microbiome as a pivotal participant in the oncogenic process rather than a passive bystander.
Experimentally, the researchers leveraged sophisticated genetic models that allowed temporal and spatial modulation of ATF6 activity specifically in colonic epithelium. Through such models, they dissected the causative role of ATF6 activation on lipid pathways without confounding systemic effects. These precise manipulations unveiled a mechanistic pathway whereby ATF6 upregulates key lipid metabolic enzymes, including those involved in de novo lipogenesis and fatty acid desaturation, thereby sculpting the lipid environment that enables microbial adaptation.
On the microbial side, analyses showed enrichment of bacterial species with enhanced lipolytic enzymes and transporters, suggesting an evolutionary advantage in lipid-rich tumor niches. Some microbes demonstrated gene expression profiles indicative of membrane remodeling enzymes, allowing them to withstand the altered physicochemical properties of the tumor microenvironment. These findings conceptualize tumor-associated microbiota not merely as a collection of organisms in proximity but as metabolic collaborators whose features co-evolve with tumor cell adaptations.
Importantly, this ATF6-lipid-microbe axis also has implications for treatment resistance. Tumor cells’ metabolic remodeling can confer resistance to therapies, and the supporting microbiota may further fortify this resilience through protective metabolite production and immune modulation. Understanding this tripartite interaction opens the door to novel combinatorial strategies that simultaneously target tumor metabolic pathways, microbial ecology, and immune responses, potentially enhancing treatment efficacy.
The clinical relevance extends to diagnostic and prognostic arenas. Alterations in colonic lipid profiles or shifts in microbial community composition governed by ATF6 activity could serve as biomarkers for tumor progression or response to therapy. Non-invasive sampling of colonic metabolites or microbial DNA might allow clinicians to monitor these signatures, providing a real-time snapshot of tumor-microbe metabolic dynamics with implications for personalized medicine.
Moreover, this research invites reconsideration of lifestyle and dietary influences on cancer and the microbiota. Given that lipid metabolism is tightly linked to dietary fat intake and systemic metabolic states, it raises provocative questions about whether interventions aimed at lipid intake or metabolic modulation could indirectly influence tumor-associated microbial adaptation and ultimately, cancer outcomes.
Mechanistically, the study elucidates a previously unappreciated signaling cascade stemming from ATF6 activation that intersects with key lipid biosynthetic regulators such as SREBP1 and PPAR pathways. These molecular interactions coordinate the metabolic shift, highlighting potential pharmacological targets. Small molecule inhibitors or modulators that temper ATF6 signaling or downstream lipid metabolic enzymes might disrupt the supportive tumor niche and microbial adaptation.
The study’s multidisciplinary approach, integrating lipid biochemistry, microbiology, oncology, and immunology, reflects the complexity of modern cancer research. It underscores the importance of viewing tumors as ecosystems whose behavior and treatment response depends on a confluence of cellular and microbial factors, metabolic networks, and molecular signaling pathways.
As research continues, understanding how widespread this ATF6-mediated lipid remodeling and microbial adaptation is across various cancer types and anatomical sites will be crucial. Early evidence suggests that similar mechanisms may operate beyond the colon, suggesting a common axis of tumor-host-microbe metabolic interactions that could redefine therapeutic approaches.
In conclusion, the activation of ATF6 in colonic tumors appears to initiate a chain of metabolic events that remodel the lipid landscape of the tumor microenvironment, promoting a symbiotic microbial adaptation that feeds back into tumor progression and therapy resistance. These discoveries pivotally expand our conceptual frameworks of tumor biology, casting light on the intertwined metabolic fates of cancer cells and their microbial inhabitants, and heralding a new frontier in oncology where metabolism and microbiology converge for transformative treatments.
Subject of Research:
Activation of the transcription factor ATF6 alters lipid metabolism in colonic tumor cells, resulting in adaptive metabolic remodeling of tumor-associated microbial communities.
Article Title:
ATF6 activation alters colonic lipid metabolism causing tumour-associated microbial adaptation.
Article References:
Coleman, O.I., Sorbie, A., Riva, A. et al. ATF6 activation alters colonic lipid metabolism causing tumour-associated microbial adaptation. Nat Metab (2025). https://doi.org/10.1038/s42255-025-01350-6
Image Credits:
AI Generated
Tags: adaptive responses of tumor-associated microbesATF6 activation and tumor biologycancer progression and lipid alterationsendoplasmic reticulum stress and cancergroundbreaking cancer research findingslipid metabolism in colon cancermetabolic plasticity in tumorsmetabolic reprogramming in cancer cellsmicrobial changes in tumor microenvironmentmicrobiome interactions in colorectal cancertherapeutic interventions targeting cancer and microbiometranscription factors in cancer research