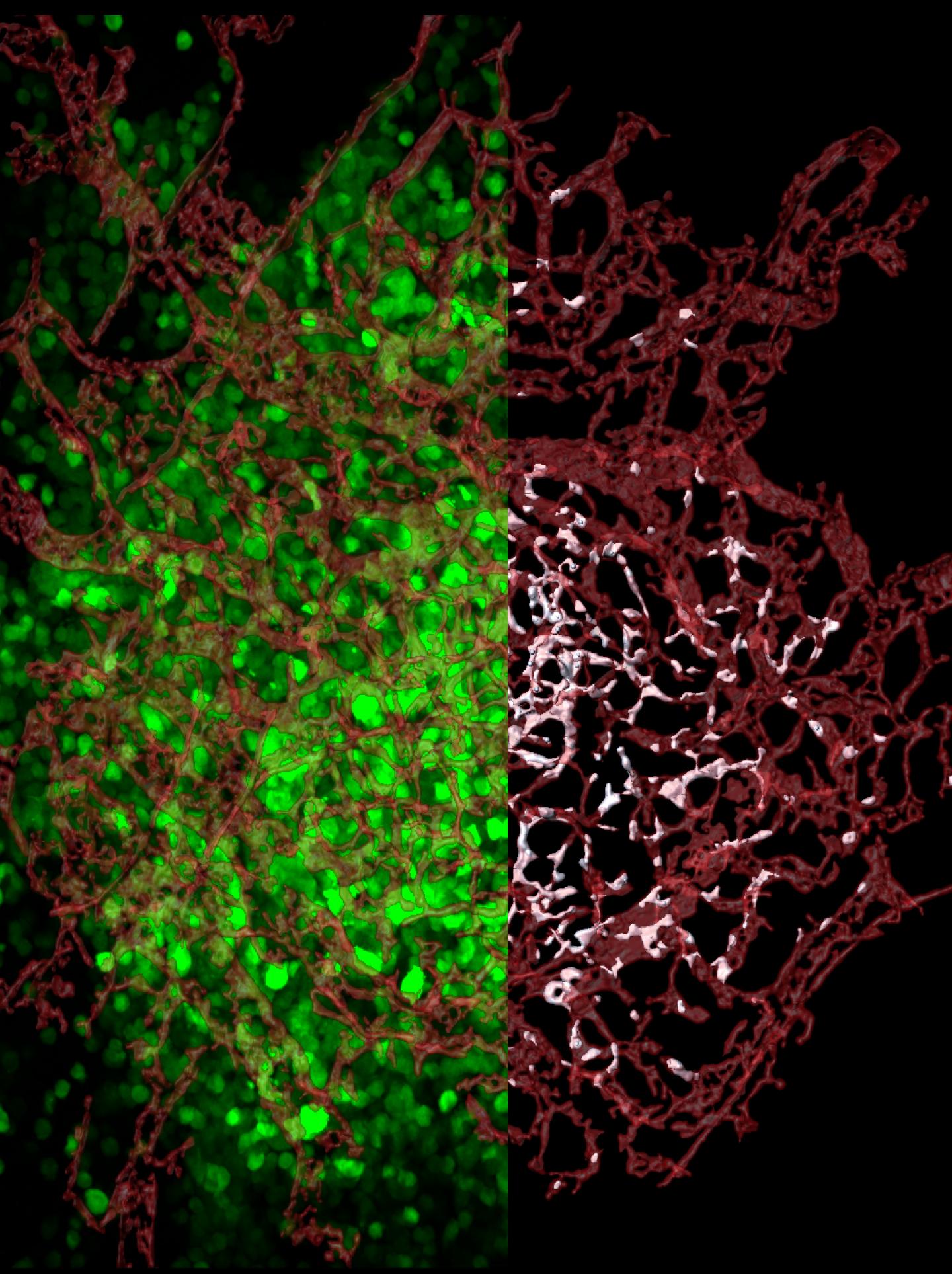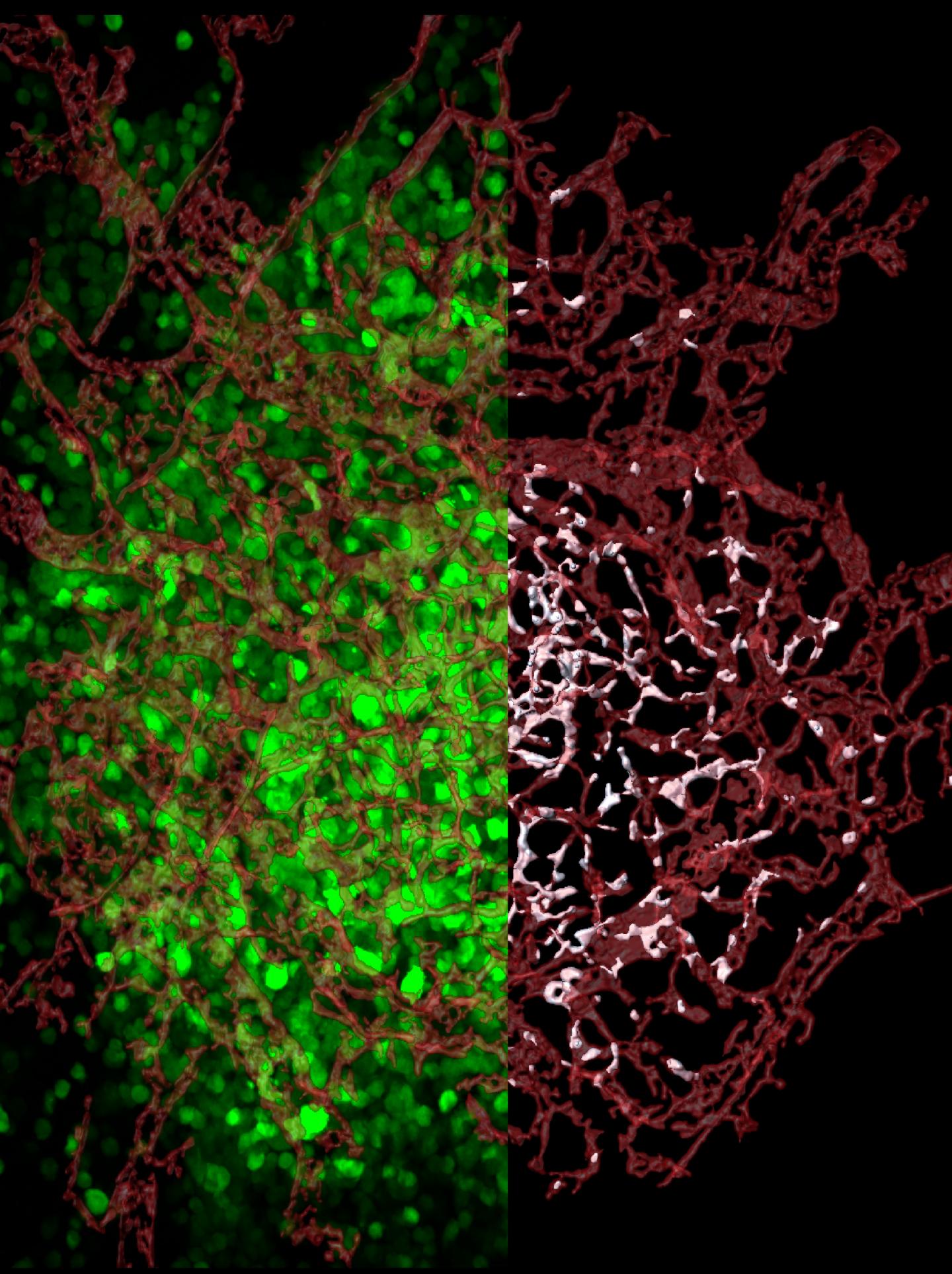
Credit: Elena Deryugina and William Kiosses
LA JOLLA, CA – May 2, 2017 – Even in remission, cancer looms. Former cancer patients and their doctors are always on alert for metastatic tumors. Now scientists at The Scripps Research Institute (TSRI) have discovered why some cancers may reoccur after years in remission.
The findings, published recently in the journal Cell Reports, show that invasive tumors can begin sending out tumor cells far earlier than previously thought. These escaping cells–which can enter the bloodstream before the primary tumor is detected–may seed secondary tumors that don't show up for years.
Importantly, the scientists demonstrated that the escaping tumor cells reach the bloodstream by entering blood vessels deep within the dense tumor core, upending the long-held belief that metastatic cells come from a tumor's invasive borders.
"The actual process of cancer cell dissemination via hematogenous routes is a relatively under-studied process, but we finally have an answer as to where it takes place," said TSRI Assistant Professor Elena Deryugina, who led the study in a long-term collaboration with TSRI Staff Scientist William Kiosses.
Metastasis Appears to Start Early
Doctors typically describe tumors in four stages: A solid tumor is more or less confined during stages 0 and 1, but starts invading nearby tissues at stage 2. It is believed that the tumor begins to send the cells to distant organs only after extensive invasion into the adjacent stroma (a nearby tissue or organ) at stage 3. Stage 4 is usually associated with the presence of secondary tumors described as metastases.
In their new study, the researchers wanted to take a closer look at this conventional view of cancer cell spread.
Using cancer cell lines generated from human fibrosarcoma and carcinoma tumors, the researchers found that primary tumors can send out cells early on–independent of cancer invasion into adjacent tissue. This could explain why doctors often see secondary tumors appearing earlier than they would have predicted.
This finding may also shed light on why patients with early stage tumors still have a risk of developing metastatic disease. "These metastases may have been seeded when the primary tumor was even too small to be visualized," Deryugina said.
Peering through dense primary tumors had been a roadblock in cancer studies until now, and this new discovery was possible because the researchers developed of animal models that allowed for microscopic analysis of tumor cell dissemination. Specifically, adapted mouse ear and chick embryo models let the scientists examine developing tumors through relatively thin tissue layers.
Cancer Cells Escape from Tumor Core
The new study is also the first to examine entire tumors to find out exactly where escaping cells come from. The scientists tagged human tumor cells with a florescent protein to distinguish them from the cells of a tumor-bearing animal. Using high-resolution confocal microscopy techniques spearheaded by Kiosses, the researchers mapped in 3-D all blood vessels across entire tumors, from the tumors' dense cores to their invasive tendrils.
The researchers mapped the location of every tumor cell relative to the center of the closest blood vessel–or visualized within blood vessels. This approach gave the researchers a way to finally analyze the escape process, called intravasation, and to demonstrate where intravasating cells enter blood vessels.
The researchers were surprised by what they found: The vast majority of tumor cells entered blood vessels within the tumor core, not in the invasive tendrils.
This discovery challenges the long-held assumption that tumor cells enter the bloodstream only after they invade adjacent stroma and reach tumor-converging blood vessels. Instead, the researchers found that fewer than 10 percent of escaped cells intravasated from the stroma-invading sprouts.
This makes sense, Deryugina said, because the newly formed vessels in the tumor core are ideal highways for escaping tumor cells.
A 2015 study by Deryugina and TSRI colleagues showed that these new vessels in the tumor core are structurally sound but permeable enough to give tumor cells a chance to slip into the bloodstream for a ride to other areas of the body. In contrast, blood vessels converging on the tumor border are less welcoming–their walls are more mature and almost impossible for tumor cells to break through.
Deryugina said it has been extremely satisfying to finally discover the primary location where tumor cells intravasate, even if the data contradict conventional cancer models.
This finding could be important for cancer patients. The research suggests a primary tumor does not have to be highly invasive to seed metastases. In fact, doctors may want to reconsider the time frame for the onset of cancer cell dissemination. While invasive tumors are more likely to manifest intravasation, the two processes–intravasation and invasion–appear to be independent of each other.
The researchers also found that levels of a protein called EGFR could be a good indicator of whether tumor cells would intravasate. EGFR appeared to regulate a tumor's ability to induce blood vessels that support cancer cell escape. "Therefore, the data indicate the importance of harnessing the EGFR activity early on in cancer patients," said Deryugina.
Next, the researchers plan to investigate the functional roles of different cell types within a primary tumor, such as inflammatory leukocytes, which also may be critically important for supporting intratumoral cancer cell intravasation.
###
The study, "Intratumoral Cancer Cell Intravasation Can Occur Independent of Invasion into the Adjacent Stroma," was supported by the National Institutes of Health (grants R01CA105412, R01CA129484 and R01CA157792).
About The Scripps Research Institute
The Scripps Research Institute (TSRI) is one of the world's largest independent, not-for-profit organizations focusing on research in the biomedical sciences. TSRI is internationally recognized for its contributions to science and health, including its role in laying the foundation for new treatments for cancer, rheumatoid arthritis, hemophilia, and other diseases. An institution that evolved from the Scripps Metabolic Clinic founded by philanthropist Ellen Browning Scripps in 1924, the institute now employs more than 2,500 people on its campuses in La Jolla, CA, and Jupiter, FL, where its renowned scientists–including two Nobel laureates and 20 members of the National Academies of Science, Engineering or Medicine–work toward their next discoveries. The institute's graduate program, which awards PhD degrees in biology and chemistry, ranks among the top ten of its kind in the nation. In October 2016, TSRI announced a strategic affiliation with the California Institute for Biomedical Research (Calibr), representing a renewed commitment to the discovery and development of new medicines to address unmet medical needs. For more information, see http://www.scripps.edu.
Media Contact
Madeline McCurry-Schmidt
[email protected]
858-784-9254
@scrippsresearch
http://www.scripps.edu
############
Story Source: Materials provided by Scienmag